REVIEW ARTICLE
Role of intestinal microecology in the regulation of energy metabolism by dietary polyphenols and their metabolites
Shaoling Lin1#, Zhengyu Wang1#, Ka-Lung Lam2, Shaoxiao Zeng1, Bee K. Tan3* and Jiamiao Hu1*
1College of Food Science, Fujian Agriculture and Forestry University, Fuzhou, China; 2School of Life Sciences, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China; 3Departments of Cardiovascular Sciences, Health Sciences and Leicester Diabetes Centre, College of Life Sciences, University of Leicester, University Road, Leicester, United Kingdom
Popular scientific summary
- Dietary polyphenols have an important impact on energy metabolism.
- Dietary polyphenols may affect host energy metabolism via regulating intestinal microecology.
- Crosstalk between polyphenols and gut microbiota may have regulatory effects on host metabolic control.
Abstract
Background: Polyphenols are a class of plant secondary metabolites with a variety of physiological functions. Polyphenols and their intestinal metabolites could greatly affect host energy metabolism via multiple mechanisms.
Objective: The objective of this review was to elaborate the role of intestinal microecology in the regulatory effects of dietary polyphenols and their metabolites on energy metabolism.
Methods: In this review, we illustrated the potential mechanisms of energy metabolism regulated by the crosstalk between polyphenols and intestinal microecology including intestinal microbiota, intestinal epithelial cells, and mucosal immune system.
Results: Polyphenols can selectively regulate the growth of susceptible microorganisms (eg. reducing the ratio of Firmicutes to Bacteroides, promoting the growth of beneficial bacteria and inhibiting pathogenic bacteria) as well as alter bacterial enzyme activity. Moreover, polyphenols can influence the absorption and secretion of intestinal epithelial cells, and alter the intestinal mucosal immune system.
Conclusion: The intestinal microecology play a crucial role for the regulation of energy metabolism by dietary polyphenols.
Keywords: polyphenols; gut microecology; energy metabolism.
Citation: Food & Nutrition Research 2019, 63: 1518 - http://dx.doi.org/10.29219/fnr.v63.1518
Copyright: Food & Nutrition Research 2019. © 2019 Shaoling Lin et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 16 July 2018; Revised: 1 December 2018; Accepted: 18 December 2018; Published: 14 February 2019
Competing interests and funding: The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
*Bee K. Tan, Departments of Cardiovascular Sciences, Health Sciences and Leicester Diabetes Centre, College of Life Sciences, University of Leicester, Leicester, United Kingdom, Email: bee.k.tan@leicester.ac.uk
*Jiamiao Hu, College of Food Science, Fujian Agriculture and Forestry University, Fuzhou, China, Email: jiamiao.hu@fafu.edu.cn; jiamiao.hu@fafu.edu.cn
#Contributed equally to this work
Polyphenols are plant secondary metabolites that widely exist in vegetables and fruits with potential contribution to the prevention of chronic diseases, including cardiovascular disease, cancer, obesity, and diabetes (1, 2). A number of polyphenols are minimally absorbed, and the rest are transformed by intestinal bacteria into other bioactive polyphenol metabolites. These polyphenols and their metabolites can influence the type and quantity of intestinal microbial species which in return may affect their bioavailability and bioactivity.
Recent findings also suggest the relationship between polyphenols and the intestinal flora in the development of obesity and obesity-related metabolic diseases. Intestinal bacterial modulation was shown to trigger obesity in both humans and animals (3, 4), and higher ratio of Firmicutes and Bacteroides phyla was found to be correlated with increased body weight (5, 6). Recent studies also revealed the selective growth-stimulating effect of gut microbes by polyphenols, leading to obesity prevention (7, 8). Therefore, polyphenols, a potential ‘metabolic prebiotics’, could provide beneficial effects to hosts (such as weight loss) by reshaping the gut microbial communities (9). In this review, we summarized recent studies investigating the effects of dietary polyphenols and their metabolites to gut microecology and energy metabolism.
Intestinal microecology and energy metabolism
The ‘intestinal microecology’ consists of three parts: intestinal microbiota, intestinal epithelial cells, and mucosal immune system that together form the intestinal mucosal barrier (10). The intestinal flora may serve the most important roles in intestinal microecology. At least 500–1,000 different bacterial species have been identified to be present in the human gastrointestinal tract, and up to 98% of intestinal flora can be classified into four phyla: Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), or Actinobacteria (3%) (11–13). Intestinal dysbiosis is considered as an important factor inducing metabolic diseases including obesity, chronic inflammation and insulin resistance, secondary to dietary changes (14–16). On the other hand, the roles of intestinal epithelial cells in the intestinal microecology cannot be overlooked. For example, secretory mucin, lysozyme, and defensins could inhibit the growth of certain intestinal microbes and prevent their intestinal adhesion; meanwhile, these secreted protein/peptides are also associated with the release of interleukin factors including IL-1α, IL-1β, IL-6, IL-8, and IL-10, which are all involved in host inflammatory response, adipose tissue energy metabolic disorder and development of insulin resistance (10). Finally, the intestinal mucosal immune system, one of the major immune organs, functions to exclude and provide tolerance to antigens (17). It has been reported that long-term intake of high-fat diets will increase the permeability of the intestinal mucosa, resulting in endotoxemia, causing chronic inflammation, and eventually inducing metabolic disorders including obesity and insulin resistance (18). The increase of mucosal permeability was also found to be positively correlated with the degree of steatosis and fat accumulation in the liver (19). Taken together, the intestinal microecology plays multiple and yet important roles in the regulation of energy metabolism.
The absorption and metabolism pathway of polyphenols in the intestine
Plant-based foods contain polyphenols in both soluble and insoluble-bound forms. As shown in Fig. 1, soluble polyphenols are mainly found in the vacuole. Dietary intake of free and soluble polyphenols can be rapidly absorbed by active transport or passive diffusion and distributed throughout the body, bringing health benefits such as oxidative inhibition of low-density lipoprotein (LDL), cholesterol and liposomes (20, 21). In contrast, insoluble polyphenols are structurally bound with proteins, cellulose, pectin, and other macromolecules in the cell wall via ether, ester or C-C bonds and released as phenolic glycosides by colonic microflora or enzymes to exert their health benefits (22–24). In fact, insoluble and high molecular weight polyphenols, which account for approximately 90–95% of the total polyphenols intake, are metabolized by gut microflora rather than being absorbed by the gastrointestinal tract (25, 26). As a consequence, a myriad of diverse groups of dietary polyphenol-derived metabolites are found in human and animal excrement (feces or urine), as shown in Table 1. Taking anthocyanin as an example, it undergoes extensive metabolism in the body before being excreted; the proportion of intact anthocyanin excreted in urine was estimated to be lower than 0.1% of the intake (Fig. 2).
Table 1. Metabolites of phenolics compounds via gut microbiota in vivo or in vitro
| Polyphenols |
Type of Study |
Metabolites |
References |
| Baicalin |
In vitro study (humans feces) |
Baicalein |
(27) |
| Epicatechin |
In vitro study (humans feces) |
(-)-5-(3’,4’-dihydroxyphenyl)-γ-valerolactone,5-(3,4-dihydroxyphenyl)-γ-valeric acid,3-(3-hydroxyphenyl)propionic acid,4-hydroxyphenylacetic acid |
(28) |
| Apigenin |
Animal study (urine) |
P-hydroxyphenylacetic acid, P-hydroxycinnamic acid,P-hydroxybenzoic acid |
(29) |
| Quercetin |
Animal study (urine) |
4-ethylphenol, Benzoic acid,4-ethylbenzoic acid |
(30) |
| Catechin |
Human intervention (urine) |
(-)-5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone(M4),(-)-5-(3′,4′-dihydroxyphenyl)-γ-valerolactone |
(31) |
| Naringenin |
In vitro study (rat feces) |
Phenylacetic acid, P-hydroxyphenylacetic acid, Protocatechuic acid |
(32) |
| Naringin |
In vitro study (humans feces) |
3-(4-hydroxyphenyl)-propionic acid,3-phenylpropionic acid |
(33) |
| Rutin |
In vitro study (humans feces) |
3-(3-hydroxyphenyl)-propionic acid,3-hydroxyphenylacetic acid |
(33) |
| Rutin |
Escherichia coli |
3,4-dihydroxyphenylacetic acid |
(34) |
| Daidzein |
In vitro study (rat feces) |
Dihydrodaidzein |
(35) |
| Anthocyanin |
In vitro study (humans feces) |
Gallic, syringic and p-coumaric acids. |
(36) |
| Chlorogenic acid |
In vitro study (humans feces) |
3-(3-hydroxyphenyl)-propionic acid |
(33) |
| Caffeic acid |
In vitro study (humans feces) |
Hydroxyphenylpropionic and Benzoicacids |
(37) |
| Ferulaic acid |
Lactobacillus and Bifidobacterium |
Coumaric acid and Caffeic acid |
(38) |
| Ellagic acid |
In vitro study (humans feces) |
Urolithin(A) |
(39) |
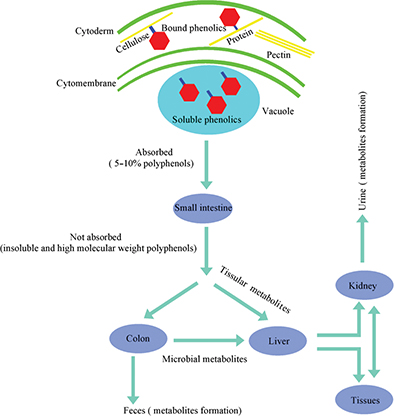
Fig. 1. The metabolic pathway of dietary polyphenols in humans. A small portion of polyphenols are directly absorbed by the small intestine. The majority of polyphenols (the insoluble and high molecular weight polyphenols) undergo extensive metabolism by gut microflora or tissues before being excreted, which represents at least 90–95% of the polyphenol intake.
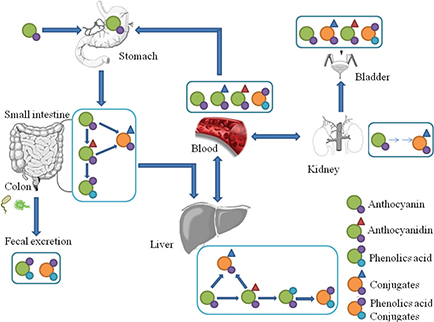
Fig. 2. The hypothetic pathways of anthocyanin absorption and metabolism based on literature review (40, 41). Anthocyanin undergoes extensive metabolism in the body; the stomach exhibited only native anthocyanin, while in other organs native anthocyanin and its metabolites (phenolic acid or conjugates) were detected before being excreted.
Energy metabolism regulatory mechanisms involving dietary polyphenols and intestinal bacteria
Polyphenols reshape the composition and diversity of gut bacterial communities
The metabolism of polyphenols by gut bacteria involves hydrolysis of glycosidic bonds and decomposition of polyphenol heterocycle (42). Glycans, the products of glycoside cleavage, are essential nutrients for most intestinal microbes (43). Evidence suggests that dietary polyphenols play a crucial role in modulating the gut microbial community such as alleviating pathogen growth, regulating commensal bacteria and probiotics, and enhancing host-microbial interactions, ultimately leading to beneficial effects such as weight loss (9, 44).
The gut microbiota is dominated by anaerobic bacteria, mainly the Firmicutes and Bacteroidetes phyla. A reduced Firmicutes-to-Bacteroidetes ratio has been associated with improved glucose levels, alleviated fat accumulation and decreased body weight (45–48). The intake of fruits and vegetables, such as apples, pears, strawberry, grapefruit, eggplant, green pepper, all of which are rich in polyphenols, may promote weight loss in obese patients. These effects were possibly attributed to the ratio of Firmicutes/Bacteroidetes lowering caused by the regulation of phenolic compounds (Fig. 3). A human intervention study performed with the administration of de-alcoholized red wine, an excellent source of anthocyanins, revealed a significant lowering of blood pressure, serum triglycerides (TG), and high-density lipoprotein (HDL) cholesterol level, which may be partly due to the greater reduction in Firmicutes than Bacteroidetes (51). It has also been found that quercetin administration led to a reduction in the Firmicutes/Bacteroidetes ratio and this was associated with reduced body weight gain and serum insulin levels in patients who consumed high-fat and high-sucrose diets (52). Similarly, Zhao et al. (53) found that the combined actions of the polyphenols quercetin and resveratrol lowered the Firmicutes/Bacteroidetes ratio in rats fed with high-fat diets, thereby decreasing their subsequent weight gain. Thus, diets containing different polyphenols might reshape the gut microbiota in various ways; however, the reduction in the ratio of Firmicutes to Bacteroidetes resulting from polyphenol administration might contribute to weight loss in obese individuals and aid in maintaining a normal body weight.
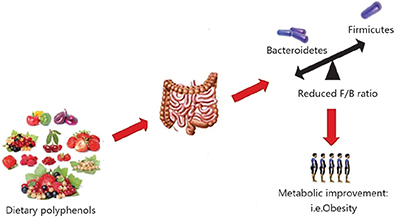
Fig. 3. Dietary polyphenols inhibit the metabolic disease related to obesity by regulating the intestinal microflora ecology, for example, lowering Firmicutes/Bacteroidetes ratio (49, 50).
Probiotics intake is also found to be correlated with weight loss (54–58). Polyphenols are known to alter a number of Bifidobacterium and Lactobacillus in the intestinal tract. For example, flavanols promoted the growth of Lactobacillus spp. and Bifidobacterium spp., which may partly be responsible for the observed reductions in plasma concentrations of C-reactive protein (CRP) (59); CRP is produced by adipose tissue and largely occurs under the transcriptional control of interleukin-6 (60, 61). In addition, these changes in Bifidobacterium and Lactobacillus abundance are also associated with significant reduction in plasma triacylglycerol level, which may contribute to the benefits associated with dietary polyphenols (62–64). In addition, polyphenol-rich pomegranate peel extract was found to increase the caecal pool of Bifidobacteria accompanied with reduced serum level of total cholesterol (TC) and LDL cholesterol induced by high-fat diet in mice (65). Another research group observed a significant increase in the proportion of Bifidobacterium in obese patients after consumption of red wine polyphenol for 4 weeks, and reported that Bifidobacterium positively correlated with HDL cholesterol levels (51). Thus, polyphenols have the ability to promote the growth of probiotic bacteria, contribute to the improvement of intestinal barrier function and prevent or treat metabolic syndrome and obesity.
Enterobacter genus (proteobacteria phylum), presented at higher baseline level in obese compared to healthy subjects, has been found to induce obesity and metabolic syndrome in human hosts (66). For example, Enterobacter cloacae produces endotoxins, causing non-obese aseptic mice to develop severe obesity, inducing inflammation and insulin resistance in mice, downregulating genes involved in fat catabolism, and activating lipogenesis genes (67, 68). Till now, available experimental evidence showed that polyphenols have an inverse relationship with intestinal Enterobacter. The promotion of Enterobacter was strongly inhibited by the presence of tea phenolics (epicatechin, catechin, 3-O-Me gallic acid, gallic acid, caffeic acid, and so on) as well as their aromatic metabolites including 3-(4-OH phenyl) propionic acid, 3-Phenylpropionic acid, and 4-OH phenylacetic acid (69). Moreover, consumption of polyphenol-containing red wine or de-alcoholized red wine normalized the Enterobacter and improved blood pressure and blood glucose dysregulation in patients with the metabolic syndromes (70). Furthermore, a combination of quercetin and resveratrol can cease the relative population increase of Enterobacter cloacae induced by high-fat diet, and this may relate to the lowering of body weights, serum lipids, and inflammatory cytokines levels (53). Thus, lowering of the relative Enterobacter population in the human intestinal tract may serve as another mechanism for polyphenols to rectify metabolic abnormalities. Enterobacter could be the new target for the prevention and treatment of obesity and related diseases.
Akkermansia muciniphila (A. muciniphila), a member of the Verrucomicrobia phylum, is believed to have anti-inflammatory and anti-obesity effects in humans and rodents (71, 72). The proportion of A. muciniphila is found to be around 3–5% in human digestive tract, but significantly reduced in obese individuals (73, 74). A. muciniphila could increase the thickness of intestinal walls by stimulating the secretion of mucin, which hinders food absorption (74). A lower relative abundance of A. muciniphila tends to induce poor performance in obesity-associated metabolic phenotypes such as insulin resistance, inflammation, and ponderal growth (75–77). As an obligate anaerobe, A. muciniphila indeed is susceptible to the presence of free oxygen radicals. Intriguingly, unabsorbed polyphenols could scavenge reactive oxygen species, thereby allowing A. muciniphila to thrive (78, 79). Some recent literature further supported this hypothesis; an in vivo study showed ellagic acid (a metabolite of pomegranate ellagitannins) promoted the growth of A. muciniphila (80). Polyphenol-rich cranberry extract was also found to improve insulin tolerance and attenuate intestinal inflammation in mice fed with high-fat/high-sucrose diet, and these effects are linked to the expansion of Akkermansia population (81). Consequently, dietary polyphenols very likely play an important role in modulating the relative abundance of A. muciniphila and therefore the control of host energy metabolism. Nevertheless, this link between changes in A. muciniphila population and weight loss awaits further experimental confirmation.
Polyphenols are metabolized to generate short-chain fatty acids via intestinal microbiota
The short-chain fatty acids (SCFAs) are beneficial for the prevention of obesity-related metabolic diseases (82, 83). Acetic acid is the major product of intestinal saccharolytic fermentation, which reduces appetite, and can be absorbed and utilized by peripheral tissues in the host (84). Propionic acid, one of the major fermentation products by Bacteroides, is further decomposed and metabolized in the liver after being absorbed into the blood, regulating the conversion of pyruvate to glucose and potentially inhibiting fat synthesis (85). Butyric acid, one of the major fermentation products by Firmicutes, is the primary energy source of colon epithelial cells (86). It is worth emphasizing that a large proportion of dietary polyphenols may be metabolized in the colon, and broken down into small molecules, including organic acids such as lactate, succinate, pyruvate, butyrate, fumarate, and acetate (87, 88). According to Bleut et al. (89), anaerobic bacteria in gut can cleave the ring structure of several flavonoids into hydroxyphenylacetic and hydroxyphenylpropionic acids, as well as into acetate and butyrate. Coincidentally, supplementation of quercetin and fructooligosaccharides enhanced the production of SCFAs, especially butyric acid, whereas supplementation of catechin and fructooligosaccharides significantly increased the production of propionic acid compared to administration of fructooligosaccharides alone (90). Besides, anthocyanins, a compound being used prophylactically and therapeutically, is also found to exhibit positive effects on the production of SCFAs, including acetic, propionic, and butyric acids, by regulating the intestinal microbial flora (82). In addition, polyphenols can induce changes in gut microbiota and therefore the production of SCFAs, leading to an upregulation of phosphorylated AMP-activated protein kinase (91, 92), which takes an up-stream and yet strong part in the energy metabolic pathways.
Polyphenols influence the activity of intestinal microbial enzymes
Intestinal microbiota affects the host physiological processes via a wide range of secretory enzymes, including hydrolase, oxidoreductase, lyase and transfer enzymes to regulate host energy metabolism (77, 93). Intriguingly, dietary polyphenols also have significant effects on the intestinal microbial enzymes. For example, polyphenols can inhibit bacterial enzyme activity by metal ions (iron and cobalt) chelation, leading to altered microbial metabolism (94, 95). The catechin epigallocatechin gallate exhibits strong antibiotic activity against Stenotrophomonas maltophilia (a kind of bacteria linked to inflammation) via inhibition of its dihydrofolate reductase (96, 97). Also, the increased abundance of Bacteroidetes by polyphenols may contribute to energy homeostasis due to the large number of glycan-degrading enzymes such as glycoside hydrolases and polysaccharide lyases possessed by Bacteroidetes (43). Thus, this might be another mechanism by which polyphenols exert weight-reducing effect via increasing Bacteroidetes abundance in gut (50).
Polyphenols influence fasting-induced adipose factor via intestinal microbiota
Fasting-induced adipose factor (Fiaf), also known as angiopoietin-like protein 4 (Angptl4), inhibits adipocytokine lipoprotein lipase activity and promotes fatty acid oxidation. Polyhenols and their metabolites can alter intestinal Fiaf expression by affecting the diversity of gut microbiota, which then can lead to the changes in lipoprotein lipase activity in gut and modulate host energy metabolism (Fig. 4). Recent evidence indicated that adding quercetin to a high-fat, high-sucrose diet significantly increased the expression of Fiaf, which was associated with beneficial changes in the gut microbiota (101); the microbial populations of Bifidobacterium and A. muciniphila were credited with the increase of intestinal Fiaf expression by secreting bioactive compounds (102, 103). For example, anthocyanin was shown to activate Fiaf expression in gut epithelium by means of increasing the growth of Bifidobacterium, and reducing fat storage (104). Furthermore, research indicated that resveratrol has the potential to attenuate mRNA expression of fatty acid synthesis genes and switch on to lipolysis-related genes in the host, which may be driven by increased Fiaf expression in the intestine (105). These changes in gene expression may be responsible for the prebiotic effect of resveratrol on the gut microbiota. In addition, the microbial metabolites of polyphenols, such as propionate and butyrate, can also promote the expression of the Fiaf in gut epithelial cell lines (106). Therefore, polyphenols and their metabolites may be able to influence intestinal Fiaf expression, and through this mechanism, regulate energy metabolism.
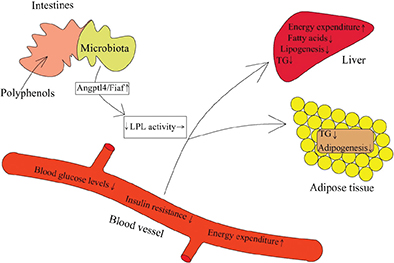
Fig. 4. Polyphenols change fiaf gene expression via reshaping microbiota structure (87, 88, 98–100). The active fiaf, an inhibitor of LPL, can promote lipid clearance in blood, suppress hepatic lipogenesis and contribute to the release of fatty acids and triacylglycerol from circulating lipoproteins in adipose tissue.
Energy metabolism regulatory mechanisms involving dietary polyphenols and intestinal mucosal epithelial cell
Polyphenols modulate glucagon-like peptide-1 secretion
Glucagon-like peptide-1 (GLP-1) is an endogenous insulinotropic peptide secreted from the intestine L cells in response to food intake (107). GLP-1 analogues promote insulin secretion and decrease glucagon secretion in a glucose-dependent manner (108). Current findings also demonstrated that resveratrol given orally exerts an anti-diabetic effect linked to increased intestinal levels of GLP-1 (109). Furthermore, anthocyanin is propitious to energy homeostasis, possibly by inducing the secretion of GLP-1 (110). In addition, polyphenol metabolites such as SCFAs in the intestine were also found to stimulate the release of GLP-1, which further inhibits appetite and food intake, delays gastric emptying and increases the sensation of fullness (87, 88, 111, 112). Therefore, the beneficial effects of polyphenols may be on the grounds of a GLP-1 receptor-dependent manner.
Polyphenols modulate sodium-coupled glucose transporter 1 expression
Sodium-coupled Glucose Transporter 1 (SGLT1), a major Na-dependent glucose co-transporter on the brush border of intestinal epithelial cells, regulates intestinal glucose uptake and glucose-dependent incretin secretion like GIP-1 (113). Polyphenols might play a significant role in controlling the dietary glucose uptake in the intestinal tract by attenuating SGLT1 expression (114, 115). Coincidentally, flavonoids, with well-documented anti-diabetic activities, can hamper glucose uptake mediated by the intestinal glucose transporter SGLT1 in mouse (116). Also, tea polyphenols have been shown to inhibit the glucose transport activity of SGLT1, with the most pronounced inhibition by epicatechin gallate (117). In addition, polyphenols include phlorizin, quercetin, kaempferol, phloretin, and cholorgenic acid, which are found to inhibit SGLT1 expression and diminish glucose responses in mice and humans (118). Streptozotocin-induced diabetic mice are given a diet containing 0.5% phloridzin for up to 14 days; blood glucose levels were significantly improved, probably through the decreased expression of SGLT1 in the small intestine (119). Based on these findings, polyphenols may act as potent inhibitors of glucose absorption by suppressing the SGLT1 sugar transporters, and serve as a promising treatment option to obesity and metabolic diseases (120, 121).
Polyphenols modulate fructose/glucose transporter expression
Experimental evidence suggested consumption of high-fructose products in rat models or in humans could lead to the development of metabolic syndrome, which is characterized by obesity, high blood pressure, and increased serum glucose, insulin and TG levels (122). Two glucose/fructose transporters (GLUT2, which transports both glucose and fructose, and GLUT5, which transports fructose only) mediate intestinal glucose/fructose transport from the intestinal lumen into enterocytes. Flavanols are the potent non-competitive inhibitors of the intestinal sugar transporters (116). Some recent evidence indicated that quercetin, apigenin, chrysin, curcumin, and bisdemethoxy can reduce the expression of GLUT2 or GLUT5 genes to interfere with fructose absorption via the intestinal–epithelial cells (123, 124). In addition, some polyphenol-rich foods were shown to improve glucose control by efficiently attenuating glucose transport across intestinal cells through interaction with the GLUT transporter family (125). In vitro studies also showed that the polyphenols contained in blackcurrant and apple extracts inhibited GLUT-mediated glucose uptake in Caco-2/TC7 cells (a frequently used cellular model of the small intestine), and cinnamon polyphenol extract can affect immune responses by regulating GLUT gene expression (126, 127). Therefore, dietary polyphenols may be beneficial to the host by regulating the rate of intestinal sugar absorption and preventing excessive glucose/fructose uptake; this may lead to reducing the risk of obesity, diabetes and the metabolic syndrome.
Energy metabolism regulatory mechanisms involving dietary polyphenols and intestinal mucosal immune system
Latest studies have correlated the impairments in intestinal immune homeostasis and the mucosal barrier with increased activation of inflammatory pathways and the pathogenesis of insulin resistance (128). Studies conducted in in vivo and in vitro models have provided evidence that polyphenol as well as polyphenol-rich foods have beneficial effects on gut health, such as modulation of mucosal immune and inflammatory response via downregulation of inflammatory cytokines and suppression of pro-inflammatory signaling pathways (129–131). Lipopolysaccharide (LPS), an endotoxin released by gram-negative bacteria, is important for the induction of gut mucosal permeability by provoking inflammatory responses and aggravating inflammation-related chronic conditions such as obesity and insulin resistance (132, 133). It has been proved that polyphenols might ameliorate the development of metabolic endotoxemia by interfering with LPS in the gut lumen (134). For instance, the supplementation of anthocyanin-rich fruit can alleviate low-grade inflammation by upregulating the interleukin-10 gene expression and downregulating inflammatory markers (interleukin-6, tumor necrosis factor-α) in the colon with an increased growth of Lactobacillus spp in the offspring (135). In addition, polyphenol metabolites showed a strong inhibition toward LPS activation. Ferulaldehyde, a water-soluble degradation product of polyphenols, inhibited the LPS-induced inflammatory response in mice (136). Urolithins, another group of gut microbiota-derived metabolites of ellagitannins, are responsible for anti-inflammatory properties (137). Also, the 3-O-methylquercetin, a metabolite of quercetin, showed stronger potential in inhibiting LPS-mediated activation of macrophage U937 cells compared to quercetin itself (138). Therefore, dietary polyphenols as well as their metabolites may act a key role in the intestinal mucosal immune system.
Conclusions
Current evidence has strongly supported the correlation of the occurrence of obesity with a shift in intestinal microecology. Polyphenols and their diverse metabolites have profound influence on the diversity and complexity of the intestinal microflora. Various studies have been carried out to understand the response of the gut microbiota with polyphenol administration as well as to identify the key microorganisms involved. It is clear that dietary polyphenols and their metabolites contribute to the maintenance of energy homeostasis and gut health through modulation of the gut microbiome, intestinal epithelial cellular function, and the mucosal immune system. Although the detailed mechanism by which polyphenols interact with the gut microecology is still not yet well characterized, polyphenols appear to influence energy metabolism and promote weight loss by re-structuring the intestinal microecology. This may provide a new viewpoint for obesity treatment via polyphenol interventions.
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (81703065), Natural Science Foundation of Fujian Province (2016J05067), Research Fund for Taiwan-Straits Postdoctoral Exchange Program (2018B003), and the China Postdoctoral Science Foundation (2018M63072) for financial support.
References
- Meydani M, Hasan ST. Dietary polyphenols and obesity. Nutrients 2010; 2: 737–51. doi: 10.3390/nu2070737
- Lin S, Hu J, Zhou X, Cheung PCK. Inhibition of vascular endothelial growth factor-induced angiogenesis by chlorogenic acid via targeting the vascular endothelial growth factor receptor 2-mediated signaling pathway. J Funct Foods 2017; 32: 285–95. doi: 10.1016/j.jff.2017.03.009
- Park JH, Schaller M, Crandall CS. Pathogen-avoidance mechanisms and the stigmatization of obese people. Evol Hum Behav 2007; 28: 410–14. doi: 10.1016/j.evolhumbehav.2007.05.008
- Dhurandhar NV. Contribution of pathogens in human obesity. Drug News Perspect 2004; 17: 307–13. doi: 10.1358/dnp.2004.17.5.829034
- Ismail NA, Ragab SH, Elbaky AA, Shoeib ARS, Alhosary Y, Fekry D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch Med Sci 2011; 7: 501–7. doi: 10.5114/aoms.2011.23418
- Koliada A, Synko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol 2017; 17: 120. doi: 10.1186/s12866-017-1027-1
- Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrino 2011; 7: 639–46. doi: 10.1038/nrendo.2011.126
- Blaut M, Bischoff SC. Probiotics and obesity. Ann Nutr Metab 2010; 57: 20–3. doi: 10.1159/000309079
- Valdés L, Cuervo A, Salazar N, Ruas-Madiedo P, Gueimonde M, González S. The relationship between phenolic compounds from diet and microbiota: impact on human health. Food Funct 2015; 6: 2424–39. doi: 10.1039/C5FO00322A
- Mccracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol 2001; 3: 1–11. doi: 10.1046/j.1462-5822.2001.00090
- Rehman A, Sina C, Gavrilova O, Häsler R, Ott S, Baines JF, et al. Nod2 is essential for temporal development of intestinal microbial communities. Gut 2011; 60: 1354–62. doi: 10.1136/gut.2010.216259
- Huang J, Chen L, Xue B, Liu Q, Ou S, Wang Y, et al. Different flavonoids can shape unique gut microbiota profile in vitro. J Food Sci 2016; 81: H2273. doi: 10.1111/1750-3841.13411
- Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol 2012; 7: 91–109. doi: 10.2217/fmb.11.142
- Sanz Y, Santacruz A, Gauffin P. Gut microbiota in obesity and metabolic disorders. Proc Nutr Soc 2010; 69: 434–41. doi: 10.1017/S0029665110001813
- Passos MDCF, Moraes-Filho JP. Intestinal microbiota in digestive diseases. Arquivos de gastroenterologia 2017; 54: 255–62. doi: 10.1590/S0004-2803.201700000-31
- Węgielska I, Suliburska J. The role of intestinal microbiota in the pathogenesis of metabolic diseases. Acta Sci Pol Technol Aliment 2016; 15: 201–11. doi: 10.17306/J.AFS.2016.2.20
- Ouwehand A, Isolauri E, Salminen S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur J Nutr 2002; 41: i32–7. doi: 10.1007/s00394-002-1105-4
- Brayden DJ, Jepson MA, Baird AW. Keynote review: intestinal Peyer’s patch M cells and oral vaccine targeting. Drug Discov Today 2005; 10: 1145–57. doi: 10.1016/S1359-6446(05)03536-1
- Miele L, Valenza V, La TG, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009; 49: 1877–87. doi: 10.1002/hep.22848
- Chandrasekara N, Shahidi F. Effect of roasting on phenolic content and antioxidant activities of whole cashew nuts, kernels, and testa. J Agr Food Chem 2011; 59: 5006–14. doi: 10.1021/jf2000772
- Bondonno CP, Croft KD, Ward N, Considine MJ, Hodgson JM. Dietary flavonoids and nitrate: effects on nitric oxide and vascular function. Nut Rev 2015; 73: 216–35. doi: 10.1093/nutrit/nuu014
- Dai J, Mumper RJ. Plant phenolics. Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010; 15: 7313–52. doi: 10.3390/molecules15107313
- Lee J, Chan BLS, Mitchell AE. Identification/quantification of free and bound phenolic acids in peel and pulp of apples (Malus domestica) using high resolution mass spectrometry (HRMS). Food Chem 2017; 215: 301–10. doi: 10.1016/j.foodchem
- Russell WR, Labat A, Scobbie L, Duncan GJ, Duthie GG. Phenolic acid content of fruits commonly consumed and locally produced in Scotland. Food Chem 2009; 115: 100–4. doi: 10.1016/j.foodchem.2008.11.086
- Clifford MN. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med 2004; 70: 1103–14. doi: 10.1055/s-2004-835835
- Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuã;o MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem 2013; 24: 1415–22. doi: 10.1016/j.jnutbio.2013.05.001
- Trinh HT, Joh EH, Kwak HY, Baek NI, Kim DH. Anti-pruritic effect of baicalin and its metabolites, baicalein and oroxylin A, in mice. Acta Pharmacolo Sin 2010, 31: 718–24. doi: 10.1038/aps.2010.42
- Roowi S, Stalmach A, Mullen W, Lean MEJ, Edwards CA, Crozier A. Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans. J Agric Food Chem 2010; 58: 1296–304. doi: 10.1021/jf9032975
- Griffiths LA, Smith GE. Metabolism of apigenin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro. Biochem J 1972; 128: 901–11. doi: 10.1042/bj1280901
- Loke WM, Jenner AM, Proudfoot JM, McKinley AJ, Hodgson JM, Halliwell B, et al. A metabolite profiling approach to identify biomarkers of flavonoid intake in humans. J Nutr 2009; 139: 2309–14. doi: 10.3945/jn.109.113613
- Li C, Lee M-J, Sheng S, Meng X, Prabhu S, Winnik B, et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem Res Toxicol 2000; 13: 177–84. doi: 10.1021/tx9901837
- Serra A, Macià A, Romero M-P, Reguant J, Ortega N, Motilva M-J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem 2012; 130: 383–93. doi: 10.1016/j.foodchem.2011.07.055
- Rechner AR, Smith MA, Kuhnle G, Gibson GR, Debnam ES, Srai SKS, et al. Colonic metabolism of dietary polyphenols: influence of structure on microbial fermentation products. Free Radical Bio Med 2004; 36: 212–25. doi: 10.1016/j.freeradbiomed.2003.09.022
- Winter J, Moore LH, Dowell VR, Bokkenheuser VD. C-ring cleavage of flavonoids by human intestinal bacteria. Appl Environ Microbiol 1989; 55: 1203–8. doi: 10.0000/PMID2757380
- Rafii F, Jackson LD, Ross I, Heinze TM, Lewis SM, Aidoo A, et al. Metabolism of daidzein by fecal bacteria in rats. Comparative Med 2007; 57: 282–6. doi: 10.1080/03079450701344738
- Hidalgo M, Oruna-Concha MJ, Kolida S, Walton GE, Kallithraka S, Spencer JPE, et al. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J Agr Food Chem 2012, 60: 3882–90. doi: 10.1021/jf3002153
- Gonthier MP, Remesy C, Scalbert A, Cheynier V, Souquet JM, Poutanen K, et al. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed Pharmacother 2006; 60: 536–40. doi: 10.1016/j.biopha.2006.07.084
- Szwajgier D, Jakubczyk A. Biotransformation of ferulic acid by Lactobacillus acidophilus Ki and selected Bifidobacterium strains. Acta Sci Pol Technol Aliment 2010; 9: 45–59. doi: 10.1016/j.biortech.2010.01.086
- Cerdá B, Periago P, Espín JC, Tomásbarberán FA. Identification of Urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agr Food Chem 2005; 53: 5571–6. doi: 10.1021/jf050384i
- Fang J. Bioavailability of anthocyanins. Drug Metab Rev 2014; 46: 508–20. doi: 10.3109/03602532.2014.978080
- Fernandes I, Faria A, Calhau C, de Freitas V, Mateus N. Bioavailability of anthocyanins and derivatives. J Funct Foods 2014; 7: 54–66. doi: 10.1016/j.jff.2013.05.010
- Aura A-M, Martin-Lopez P, O’Leary KA, Williamson G, Oksman-Caldentey K-M, Poutanen K, et al. In vitro metabolism of anthocyanins by human gut microflora. Eur J Nutr 2005; 44: 133–42. doi: 10.1007/s00394-004-0502-2
- Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. P Natl Acad Sci Usa 2009; 106: 5859–64. doi: 10.1073/pnas.0901529106
- Xue B, Xie J, Huang J, Chen L, Gao L, Ou S, et al. Plant polyphenols alter a pathway of energy metabolism by inhibiting fecal Bacteroidetes and Firmicutes in vitro. Food Funct 2016; 7: 1501–7. doi: 10.1039/c5fo01438g
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. P Natl Acad Sci Usa 2005; 102: 11070–5. doi: 10.1073/pnas.0504978102
- Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008; 3: 213–23. doi: 10.1016/j.chom.2008.02.015
- Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017; 15: 73. doi: 10.1186/s12967-017-1175-y
- Kemperman RA, Gross G, Mondot S, Possemiers S, Marzorati M, Van de Wiele T, et al. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res Int 2013; 53: 659–69. doi: 10.1016/j.foodres.2013.01.034
- Serino M, Luche E, Chabo C, Amar J, Burcelin R. Intestinal microflora and metabolic diseases. Diabetes Metab 2009; 35: 262–72. doi: 10.1016/j.diabet.2009.03.003
- Rastmanesh R. High polyphenol, low probiotic diet for weight loss because of intestinal microbiota interaction. Chem-Biol Interact 2011; 189: 1–8. doi: 10.1016/j.cbi.2010.10.002
- Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, et al. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr 2012; 95: 1323–34. doi: 10.3945/ajcn.111.027847
- Etxeberria U, Arias N, Boqué N, Macarulla MT, Portillo MP, Martinez JA, et al. Reshaping faecal gut microbiota composition by the intake of trans -resveratrol and quercetin in high-fat sucrose diet-fed rats. J Nutr Biochem 2015; 26: 651–60. doi: 10.1016/j.jnutbio.2015.01.002
- Zhao L, Zhang Q, Ma W, Tian F, Shen H, Zhou M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct 2017; 8: 4644–56. doi: 10.1039/c7fo01383c
- Million M, Lagier JC, Yahav D, Paul M. Gut bacterial microbiota and obesity. Clin Microbiol Infec 2013; 19: 305–13. doi: 10.1111/1469-0691.12172
- Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroentero 2014; 20: 16079–94. doi: 10.3748/wjg.v20.i43.16079
- Sanchez M, Panahi S, Tremblay A. Childhood obesity: a role for gut microbiota? Int J Env Res Pub He 2015; 12: 162–75. doi: 10.3390/ijerph120100162
- Arora T, Singh S, Sharma RK. Probiotics: interaction with gut microbiome and antiobesity potential. Nutrition 2013; 29: 591–6. doi: 10.1016/j.nut.2012.07.017
- Chen J, He X, Huang J. Diet effects in gut microbiome and obesity. J Food Sci 2014; 79: R442–51. doi: 10.1111/1750-3841.12397
- Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JPE. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr 2011; 93: 62–72. doi: 10.3945/ajcn.110.000075
- Castell JV, Gómez-Lechón MJ, David M, Andus T, Geiger T, Trullenque R, et al. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. Febs Lett 1989; 242: 237–9. doi: 10.1016/0014-5793(89)80476-4
- Boutsikou T, Mastorakos G, Kyriakakou M, Margeli A, Hassiakos D, Papassotiriou I, et al. Circulating levels of inflammatory markers in intrauterine growth restriction. Mediat Inflamm 2010; 2010: 790605. doi: 10.1155/2010/790605
- Hage FG, Szalai AJ. C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. J Am Coll Cardiol 2007; 50: 1115–22. doi: 10.1016/j.jacc.2007.06.012
- Ridker PM, Cook NR. Biomarkers for prediction of cardiovascular events. N Engl J Med 2007; 302: 1472–3. doi: 10.1001/jama.2009.1637
- Fogliano V, Corollaro ML, Vitaglione P, Napolitano A, Ferracane R, Travaglia F, et al. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol Nutr Food Res 2011; 55: S44–55. doi: 10.1002/mnfr.201000360
- Neyrinck AM, Van Hée VF, Bindels LB, De Backer F, Cani PD, Delzenne NM. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: potential implication of the gut microbiota. Br J Nutr 2013; 109: 802–9. doi: 10.1017/S0007114512002206
- Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J 2013; 7: 880–4. doi: 10.1038/is mej.2012.153
- Keskitalo A, Munukka E, Toivonen R, Hollmén M, Kainulainen H, Huovinen P, et al. Enterobacter cloacae administration induces hepatic damage and subcutaneous fat accumulation in high-fat diet fed mice. PLoS One 2018; 13: e0198262. doi: 10.1371/journal.pone.0198262
- Yan H, Fei N, Wu G, Zhang C, Zhao L, Zhang M. Regulated inflammation and lipid metabolism in colon mRNA expressions of obese germfree mice responding to enterobacter cloacae B29 combined with the high fat diet. Front Microbiol 2016; 7: 1786. doi: 10.3389/fmicb.2016.01786
- Lee HC, Jenner AM, Low CS, Lee YK. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res Microbiol 2006; 157: 876–84. doi: 10.1016/j.resmic.2006.07.004
- Moreno-Indias I, Sánchez-Alcoholado L, Pérez-Martínez P, Andrés-Lacueva C, Cardona F, Tinahones F, et al. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct 2016; 7: 1775–87. doi: 10.1039/c5fo00886g
- Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016; 65: 426–36. doi: 10.1136/gutjnl-2014-308778
- van Passel MWJ, Kant R, Zoetendal EG, Plugge CM, Derrien M, Malfatti SA, et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS One 2011; 6: e16876. doi: 10.1371/journal.pone.0016876
- Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microb 2008; 74: 1646–8. doi: 10.1128/AEM.01226-07
- Belzer C, de Vos WM. Microbes inside–from diversity to function: the case of Akkermansia. ISME J 2012; 6: 1449–58. doi: 10.1038/ismej.2012.6
- Everard A, Lazarevic V, Derrien M, Girard M, Muccioli, GM, Neyrinck AM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011; 60: 2775–86. doi: 10.2337/db11-0227
- Everard A, Belzer C, Geurts L, Ouwerkerk, JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Pnas 2013; 110: 9066–71. doi: 10.1073/pnas.1219451110
- Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract Res Cl Ga 2017; 31: 637–42. doi: 10.1016/j.bpg.2017.10.001
- Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. Nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 2004; 54: 1469–76. doi: 10.1099/ijs.0.02873-0
- Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, et al. Dietary polyphenols promote growth of the gut bacteriumAkkermansia muciniphila and attenuate high-fat diet–induced metabolic syndrome. Diabetes 2015; 64: 2847–58. doi: 10.2337/db14-1916
- Henning SM, Summanen PH, Lee R-P, Yang J, Finegold SM, Heber D, et al. Pomegranate ellagitannins stimulate the growth of Akkermansia muciniphila in vivo. Anaerobe 2017; 43: 56–60. doi: 10.1016/j.anaerobe.2016.12.003
- Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015; 64: 872–83. doi: 10.1136/gutjnl-2014-307142
- Hu J, Lin S, Zheng B, Cheung PCK. Short-chain fatty acids in control of energy metabolism. Crit Rev Food Sci Nutr.2016; 58: 1243–9. doi: 10.1080/10408398.2016.1245650
- Yan Y, Peng Y, Tang J, Mi J, Lu L, Li X, et al. Effects of anthocyanins from the fruit of Lycium ruthenicum Murray on intestinal microbiota. J Funct Foods 2018; 48, 533–41. doi: 10.1016/j.jff.2018.07.053
- Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015; 7, 2839–49. doi: 10.3390/nu7042839
- Wright RS, Anderson JW, Bridges SR. Propionate inhibits hepatocyte lipid synthesis. Proceedings of the Society for Experimental Biology and Medicine Society 1990; 195: 26–9. doi: 10.3181/00379727-195-43113
- Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. Fems Microbiol Lett 2002; 217: 133–9. doi: 10.1111/j.1574-6968.2002.tb11467
- Bauer E, Williams BA, Smidt H, Mosenthin R, Verstegen MWA. Influence of dietary components on development of the microbiota in single-stomached species. Nutr Res Rev 2006; 19: 63–78. doi: 10.1079/NRR2006123
- Bleau C, Karelis AD, St-Pierre DH, Lamontagne L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes-Metab Res 2015; 31: 545–61. doi: 10.1002/dmrr.2617
- Blaut M, Schoefer L, Braune A. Transformation of flavonoids by intestinal microorganisms. Int J Vitam Nutr Res 2003; 73: 79–87. doi: 10.1024/0300-9831.73.2.79
- Zheng C-J, Liu R, Xue B, Luo J, Gao L, Wang Y, et al. Impact and consequences of polyphenols and fructooligosaccharide interplay on gut microbiota in rats. Food Funct 2017; 8: 1925–32. doi: 10.1039/c6fo01783e
- Hara H, Orita N, Hatano S, Ichikawa H, Hara Y, Matsumoto N, et al. Effect of tea polyphenols on fecal flora and fecal metabolic products of pigs. J Vet Med Sci 1995; 57: 45–9. doi: 10.1292/jvms.57.45
- Henning SM, Yang J, Hsu M, Lee R-P, Grojean EM, Ly A, et al. Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur J Nutr 2018; 57: 2759–69. doi: 10.1007/s00394-017-1542-8
- Kaminsky LS, Zhang Q-Y. The small intestine as a xenobiotic-metabolizing organ. Drug Metab Dispos 2003; 31: 1520–5. doi: 10.1124/dmd.31.12.15290
- Mcdonald M, Mila I, Scalbert A. Precipitation of metal ions by plant polyphenols: optimal conditions and origin of precipitation. J Agr Food Chem 1996; 44: 599–606. doi: 10.1021/jf950459q
- Smith AH, Zoetendal E, Mackie RI. Bacterial mechanisms to overcome inhibitory effects of dietary tannins. Microbial Ecol 2005; 50: 197–205. doi: 10.1007/s00248-004-0180-x
- Matera G, Barreca GS, Puccio R, Quirino A, Liberto MC, De RM, et al. Stenotrophomonas maltophilia lipopolysaccharide (LPS) and antibiotics: ‘in vitro’ effects on inflammatory mediators. Infez Med 2004; 12: 227–38.
- Navarro-Martínez MD, Navarro-Perán E, Cabezas-Herrera J, Ruiz-Gómez J, García-Cánovas F, Rodríguez-López JN. Antifolate Activity of Epigallocatechin Gallate against Stenotrophomonas maltophilia. Antimicro Agents 2005; 49: 2914–20. doi: 10.1128/AAC.49.7.2914-2920.2005
- Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res 2010; 51: 1101–12. doi: 10.1194/jlr.M002774
- Zhang L, Carmody RN, Kalariya HM, Duran RM, Moskal K, Poulev A, et al. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. J Nutr Biochem 2018; 56: 142–51. doi: 10.1016/j.jnutbio.2018.02.009
- Shen W, Gaskins HR, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J Nutr Biochem 2014; 25: 270–80. doi: 10.1016/j.jnutbio.2013.09.009
- Moreno MF, Anhê FF, Nachbar R, St-Pierre P, Oyama LM, Marette A. Effect of synergism between leucine and quercetIn: a new strategy against metabolic disorders. Isanh 2016; 3. doi: 10.18143/JISANH_v3i4_1342
- Pham P, Cotten R, Kolinek T, Parker S, Vattem D, Maitin V. Preliminary characterization of secreted bioactive compounds from Bifidobacterium longum with modulatory activity towards enterocytic Fasting Induced Adipocyte Factor (FIAF). Faseb J 2012; 26(1_supplement): 373.6.
- Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, et al. Differential Modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. Mbio 2014; 5: e01438–14. doi: 10.1128/mBio.01438-14
- Jamar G, Estadella D, Pisani LP. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. BioFactors 2017; 43: 507–16. doi: 10.1002/biof.1365
- Qiao Y, Sun J, Xia S, Tang X, Shi Y, Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct 2014; 5: 1241–9. doi: 10.1039/c3fo60630a
- Grootaert C, Van de Wiele T, Van Roosbroeck I, Possemiers S, Vercoutter-Edouart A-S, Verstraete W, et al. Bacterial monocultures, propionate, butyrate and H2O2 modulate the expression, secretion and structure of the fasting induced adipose factor in gut epithelial cell lines. Environ Microbiol 2011; 13: 1778–89. doi: 10.1111/j.1462-2920.2011.02482.x
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. P Natl Acad Sci Usa 2009; 106: 1285–90. doi: 10.1073/pnas.0806720106
- Panickar KS. Effects of dietary polyphenols on neuroregulatory factors and pathways that mediate food intake and energy regulation in obesity. Mol Nutr Food Res 2013; 57: 34–47. doi: 10.1002/mnfr.201200431
- Dao TMA, Waget A, Klopp P, Serino M, Vachoux C, Pechere L, et al. Resveratrol increases glucose induced GLP-1 secretion in mice: a mechanism which contributes to the glycemic control. PLoS One 2013; 6: e20700. doi: 10.1371/journal.pone.0020700
- Kato M, Tani T, Terahara N, Tsuda T. The anthocyanin delphinidin 3-rutinoside stimulates glucagon-like peptide-1 secretion in murine GLUTag cell line via the Ca21/calmodulin-dependent kinase II pathway. PLoS One 2015; 11: e0126157. doi: 10.1371/journal.pone.0126157
- Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes 2015; 39: 424–9. doi: 10.1038/ijo.2014.153
- Yadav H, Lee J-H, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via Butyrate-induced GLP-1 hormone secretion. J Biol Chem 2013; 288: 25088–97. doi: 10.1074/jbc.M113.452516
- Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, et al. Na+-d-glucose Cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012; 61: 187–96. doi: 10.2337/db11-1029
- Wang Z, Clifford MN, Sharp P. Analysis of chlorogenic acids in beverages prepared from Chinese health foods and investigation, in vitro, of effects on glucose absorption in cultured Caco-2 cells. Food Chem 2008; 108: 369–73. doi: 10.1016/j.foodchem.2007.10.083
- Manzano S, Williamson G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol Nutr Food Res 2010; 54: 1773–80. doi: 10.1002/mnfr.201000019
- Kwon O, Eck P, Chen S, Corpe CP, Lee JH, Kruhlak M, et al. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. Faseb J 2007; 21: 366–77. doi: 10.1096/fj.06-6620com
- Kobayashi Y, Suzuki M, Satsu H, Arai S, Hara Y, Suzuki K, et al. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agr Food Chem 2000; 48: 5618–23. doi: 10.1021/jf0006832
- Schulze C, Bangert A, Kottra G, Geillinger KE, Schwanck B, Vollert H, et al. Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol Nutr Food Res 2014; 58: 1795–808. doi: 10.1002/mnfr.201400016
- Masumoto S, Akimoto Y, Oike H, Kobori M. Dietary phloridzin reduces blood glucose levels and reverses Sglt1 expression in the small intestine in streptozotocin-induced diabetic mice. J Agr Food Chem 2009; 57: 4651–6. doi: 10.1021/jf9008197
- Schulze C, Bangert A, Schwanck B, Vollert H, Blaschek W, Daniel H. Extracts and flavonoids from onion inhibit the intestinal sodium-coupled glucose transporter 1 (SGLT1) in vitro but show no anti-hyperglycaemic effects in vivo in normoglycaemic mice and human volunteers. J Funct Foods 2015; 18: 117–28. doi: 10.1016/j.jff.2015.06.037
- Johnston K, Sharp P, Clifford M, Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. Febs Lett 2005; 579: 1653–7. doi: 10.1016/j.febslet.2004.12.099.
- Toop CR, Gentili S. Fructose beverage consumption induces a metabolic syndrome phenotype in the rat: a systematic review and meta-analysis. Nutrients 2016; 8: 577. doi: 10.3390/nu8090577
- Andrade N, Araújo JR, Correia-Branco A, Carletti JV, Martel F. Effect of dietary polyphenols on fructose uptake by human intestinal epithelial (Caco-2) cells. J Funct Foods 2017; 36: 429–39. doi: 10.1016/j.soard.2017.09.494
- Lee Y, Lim Y, Kwon O. Selected phytochemicals and culinary plant extracts inhibit fructose uptake in Caco-2 cells. Molecules 2015; 20: 17393–404. doi: 10.3390/molecules200917393
- Farrell TL, Ellam SL, Forrelli T, Williamson G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: interactions with SGLT1 and GLUT2 transporters. Biofactors 2013; 39: 448–56. doi: 10.1002/biof.1090
- Castro Acosta ML. Beneficial effects of blackcurrant and apple polyphenols on glucose homeostasis. London: King’s College London; 2017.
- Cao H, Urban JJF, Anderson RA. Cinnamon polyphenol extract affects immune responses by regulating anti- and proinflammatory and glucose transporter gene expression in mouse macrophages. J Nutr 2008; 138: 833–40. doi: 10.1093/jn/138.5.833
- Gil-Cardoso K, Ginés I, Pinent M, Ardévol A, Blay M, Terra X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr Res Rev 2016; 29: 234–48. doi: 10.1017/S0954422416000159
- Williams AR, Krych L, Fauzan Ahmad H, Nejsum P, Skovgaard K, Nielsen DS, et al. A polyphenol-enriched diet and Ascaris suum infection modulate mucosal immune responses and gut microbiota composition in pigs. PLoS One 2017; 12: e0186546. doi: 10.1371/journal.pone.0186546
- Romier B, Schneider YJ, Larondelle Y, During A. Dietary polyphenols can modulate the intestinal inflammatory response. Nutr Rev 2009; 67: 363–78. doi: 10.1111/j.1753-4887.2009.00210.x
- Farzaei M, Rahimi R, Abdollahi M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr Pharm Biotechno 2015; 16: 196–210. doi: 10.2174/1389201016666150118131704
- Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol 2013; 11: 639–47. doi: 10.1038/nrmicro3089
- Fan X, Jiao H, Zhao J, Wang X, Lin H. Lipopolysaccharide impairs mucin secretion and stimulated mucosal immune stress response in respiratory tract of neonatal chicks. Comp Biochem Phys C 2017; 204: 71–8. doi: 10.1016/j.cbpc.2017.11.011
- Wong X, Madrid AM, Tralma K, Castilo R, Carrasco-Pozo C, Navarrete P, et al. Polyphenol extracts interfere with bacterial lipopolysaccharide in vitro and decrease postprandial endotoxemia in human volunteers. J Funct Foods 2016; 26: 406–17. doi: 10.1016/j.jff.2016.08.011
- Morais CA, Oyama LM, Oliveira JLD, Garcia MC, Rosso VVD, Amigo LSM, et al. Jussara (Euterpe edulis Mart.) supplementation during pregnancy and lactation modulates the gene and protein expression of inflammation biomarkers induced by trans-fatty acids in the colon of offspring. Mediat Inflamm 2014; 2014: 987927. doi: 10.1155/2014/987927
- Radnai B, Tucsek Z, Bognar Z, Antus C, Mark L, Berente Z, et al. A water-soluble degradation product of polyphenols, inhibits the lipopolysaccharide-induced inflammatory response in mice. J Nutr 2009; 139: 291–7. doi: 10.3945/jn.108.101345
- González-Sarrías A, Larrosa M, Tomás-Barberán FA, Dolara P, Espín JC. NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Brit J Nutr 2010; 104: 503–12. doi: 10.1017/S0007114510000826
- Okoko T, Oruambo IF. Inhibitory activity of quercetin and its metabolite on lipopolysaccharide-induced activation of macrophage U937 cells. Food Chem Toxicol 2009; 47: 809–12. doi: 10.1016/j.fct.2009.01.013