ORIGINAL ARTICLE
Ethanol extracts of Allium sp. regulate cyclooxygenase-2 and E-cadherin expression in gastric cancer MKN74 cell line and enhance doxorubicin toxicity
Agnieszka Korga1, Marta Ostrowska2*, Magdalena Iwan1, Małgorzata Skierucha3,4, Aleksandra Józefczyk5, Piotr Pawłowski5, Jarosław Dudka2, Ryszard Maciejewski3† and Robert Sitarz3†
1Department of Medical Biology, Medical University of Lublin, Lublin, Poland; 2Department of Toxicology, Medical University of Lublin, Lublin, Poland; 3Department of Anatomy, Medical University of Lublin, Lublin, Poland; 4Surgical Oncology Department, Medical University of Lublin, Lublin, Poland; 5Department of Pharmacognosy with Medicinal Plant Unit, Medical University of Lublin, Lublin, Poland
Popular scientific summary
- The gastric cancer (GC) incidence remains the third most common cause of cancer-related death. During gastric carcinogenesis cadherin-1 (CDH1) down-expression and cyclooxygenase 2 (COX2) overexpression may be observed what contributes to the GC invasion, its metastases and poor prognosis.
- We aimed to investigate for the first time the chemo preventive effect of ethanol extracts from various Allium species as well as the effect on COX2 and CDH1 expression relationship in the human GC cell lines, and doxorubicin toxicity.
- Our results indicate the potential application of Allium genus to chemo prevention and treatment support through CDH1 restoration and COX2 down regulation. However, the issue needs further in vivo investigations as in the future it might be used in clinics, possibly as a supplement to the chemotherapy.
- What is more, the tested extracts revealed cytotoxicity properties against GC cell lines and they had beneficial effect on DOX treatment.
Abstract
Background: Gastric cancer (GC) remains one of the leading causes of cancer-related death. Its aetiology is multifactorial, but the major risk factor is a high in salt diet. During gastric carcinogenesis, cadherin-1 (CDH1) down-expression and cyclooxygenase 2 (COX2) overexpression may be observed. The intensity of these alterations contributes to the GC invasion, its metastases and poor prognosis. As the diet plays a significant role in the aetiology of GC, it is reasonable to include the nutritional chemoprevention agents. One of the plant genus demonstrating chemoprotective properties is Allium genus, which includes garlic. The relationship between CDH1 and COX2 in GC cells treated with Allium species extract has never been evaluated.
Methods: In this study, the MKN28 and MKN74 GC cell lines were treated with ethanol extracts of Allium angulosum L., Allium lusitanicum Lam., Allium sativum L. (from Malaysia and Poland), Allium tibeticum Rendle and Allium ursinum L. The cytotoxicity of the extracts and their influence on COX2 and CDH1 mRNA and protein expression were evaluated as well as their influence on doxorubicin’s (DOX) efficacy – a drug that has been used in GC treatment.
Results: Among the tested species, ethanol extracts of A. sativum L. (Poland and Malaysia), A. tibeticum Rendle and A. ursinum L. influenced the levels of CDH1 and COX2, but only in the MKN74 cell line. Thus, it is possible that tumours with increased COX2 expression will be more susceptible to garlic treatment. Observed phenomenon was independent of Allium extract’s toxicity. In comparison to DOX, tested extracts were more toxic. Moreover, A. sativum revealed synergistic effect with the drug.
Conclusion: In conclusion, the results indicate the potential application of Allium genus to GC chemoprevention and treatment support through CDH restoration and COX2 downregulation. This issue needs further investigations as it might be used in clinics.
Keywords: Garlic; Allium sp; gastric cancer; cyclooxygenase 2; E-cadherin
Citation: Food & Nutrition Research 2019, 63: 3449 - http://dx.doi.org/10.29219/fnr.v63.3449
Copyright: © 2019 Agnieszka Korga et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 4 February 2019; Revised: 30 April 2019; Accepted: 8 May 2019; Published: 25 June 2019
Competing interests and funding: The authors declare that they have no conflict of interests. The project was partly supported by the Ministry of Science and Higher Education grant N N402 423838 and Funds for Statutory Activity of Medical University of Lublin, Poland.
*Marta Ostrowska, Department of Toxicology, Medical University of Lublin, 8b Jaczewski Str., 20-093 Lublin, Poland, Email: martaostrowska@umlub.pl
†Authors share senior authorship
Gastric cancer (GC) remains the third most common cause of cancer-related death worldwide (1). Despite advances in diagnosis and treatment, the 5-year survival rate is only around 20% (2, 3). Its aetiology is multifactorial, but the major risk factor is high content of nitrates and high salt intake. Helicobacter pylori infection also plays an important role. GC development involves multiple genetic and epigenetic alterations (2, 3). Despite advances in diagnosis, the disease is usually detected after invasion because of nonspecific symptoms in its early stages. Doxorubicin (DOX), an anthracycline chemotherapy agent, had been used as the gold standard for advanced GC since 1980 (4). However, DOX-based treatment is not currently recommended because of the frequent development of resistance and poor drug efficacy. As the diet plays a significant role in the aetiology of GC, it is reasonable to include nutritional chemoprevention agents in the diet.
Previous studies have shown properties of phytochemical compounds beyond their antioxidant activity. It turns out that natural compounds can also affect the proliferation, growth or metastasis of tumours thus being natural anticancer drugs. The amount of phytochemical compounds of daily diet may be the basis for a valuable cancer prevention strategy. One of the genus demonstrating chemoprotective properties is Allium genus. It comprises more than 600 species and belongs to the Amaryllidaceae family. They are plants of the northern hemisphere. For most species, the place of origin is Asia as well as Africa and North America (5). The best known and used, since ancient times, plant of this species is its characteristic representative “garlic” (Allium sativum L.). Wild (Bear) garlic (A. ursinum L.) reveals similar taste and healing properties. Mouse garlic (Allium angulosum L.) is important for its use as a spice, a vegetable and an ornamental plant in India (6). This area also includes Allium tibeticum Rendle which is the synonym of A. sikkimense Baker. Allium lusitanicum Lam. is a species that grows in southern Europe and Asian area. In the last three decades, studies focusing on garlic and its compounds have provided much evidence for their ability to prevent or treat various diseases. The genus shows antibacterial, antifungal, immunostimulating, antioxidant as well as chemoprotective properties and the ability to inhibit the growth of some cancer cells (7, 8). However, molecular mediators of these activities remain an intense area of research (9). In vitro and in vivo studies have shown that garlic demonstrates chemopreventive activity against cancer by inhibiting cell proliferation, arresting the cell cycle, inducing cellular apoptosis and blocking invasion and metastasis (10, 11).
Cyclooxygenase 2 (COX2) is an isoform of COX enzymes that play an important role in the development and progression of GC (12–14). COX enzymes are induced in response to pro-inflammatory cytokines and growth factors. They are responsible for the catalysis of prostanoid biosynthesis, thus playing an important role in regulating various cellular functions under physiological and pathological conditions, including the process of tumour formation (15–17). Studies have shown that COX2 is involved in tumour invasion and metastasis, among others, by reducing the expression of cadherin E (CDH). CDH is a cell adhesion molecule whose expression decreases during tumour progression. The most important function of CDH is inhibition of tumour invasion and metastasis. Thanks to this, the normal epithelial tissue architecture is maintained. An abnormal expression and function of CDH has been observed in several epithelial cancers, including GC (18, 19). The inverse relationship between CDH1 and COX2 and its molecular mechanism in tumour cells was demonstrated for the first time in non-small cell lung cancer, in which the inhibition of tumour COX2 with celecoxib led to increased expression of CDH1 (20). A similar effect was also found in colon, bladder as well as head and neck cancer cells (21–23). For the first time, our research team demonstrated this relationship in GC (24). COX2 over-expression has been found in many types of tumours, including GC (12, 25), what contributes to its invasion, metastasis and poor prognosis (26, 27). Studies that showed COX2 upregulation in GC confirm the fact that it is reasonable to investigate the chemopreventive role of COX2 inhibitors in this cancer. Many animal and epidemiological studies have provided evidence that non-steroidal anti-inflammatory drugs and selective COX2 inhibitors suppress tumourgenesis (27–30). Previous research articles indicate that COX2 inhibitors not only exhibit chemoprophylactic effect in GC but also have chemotherapeutic potential (31). There are also many reports on the effect of COX2 inhibitors on the efficacy of anticancer drugs, but the results are not consistent (32).
Allium genus plants have been known for their anti-inflammatory properties for centuries (33). It has been proven that garlic may inhibit COX2 activity (34). For this reason, the study aimed to investigate for the first time the chemopreventive effect of ethanol extracts from various Allium species as well as their effect on COX2 and CDH1 expression in the human GC cell lines, and DOX toxicity.
Materials and methods
Plant material
Selected species of fresh Allium (bulbus and radix) were obtained from the Botanical Garden Chair and Department of Pharmacognosy with Medicinal Plant Unit, Faculty of Pharmacy, Medical University of Lublin (Poland) and included A. angulosum L. A. lusitanicum Lam., A. sativum L. (from Poland, PL), A. tibeticum Rendle and A. ursinum L. The seed came from an exchange between botanical gardens. A. angulosum L. – The Tropical Greenhaus in Ljubljana, Slovenia, A. lusitanicum Lam. – Botanischer Garten der Universiteat Graz, Austria, A. tibeticum Rendle I Grădina Botanica Jibou, Vasile Fati, Romania, A. ursinum L., A. sativum L. – Medicinal Herbs Centre, Facultas Medica – Universites Masarykiena, Brno, Czech Republic. In the study, we also used fresh (2-day) tubers and roots of Allium sativum L. purchased from a market in Singapore (Malaysia, MY). The plant material was identified by specialists from the Department. Fresh substances have been chopped to a medium crushed content and extracted with 65% ethanol. The extraction was carried out by 24-h maceration with automatic shaking (250 rpm/min) for the last hour. After extraction, the extracts were soaked and supplemented with extractors to a volume in which the ratio of the mass of plant substance (g) used to the sample volume (mL) was 1:5. Extracts were used as stock solutions.
Cell culturing and treatment
Human GC cell lines MKN28 and MKN74 were acquired from the Department of Pathology at the University Medical Center in Utrecht, Holland. The cells were maintained in RPMI medium (Corning, USA) supplemented with 10% foetal bovine serum (PAA Laboratories, Canada) in a humidified atmosphere of 5% CO2 at 37°C. Prior to the experiments, all cell lines have been tested for the presence of mycoplasma using LookOut® Mycoplasma PCR Detection Kit (Sigma Aldrich, USA). Tested extracts were added to the cell cultures in final concentration 0.3 µg/mL (ethanol concentration did not exceed 0.1% in the culture medium) and incubated for 24 and 48 h.
In the second part of the study where the influence of extracts on DOX toxicity was evaluated, the MKN74 cells were incubated with 1 μM DOX (EBEWE Pharma, Austria) and tested with extracts alone or combined (DOX + single extract). The concentration of DOX was based on reported clinically achievable plasma concentrations (35). Extract was selected on the basis of previous gene expression analysis (unpublished data) – we chose A. sativum MY and PL, A. ursinum and A. tibeticum that upregulated CHD1 and downregulated COX2 expression at the same time.
The cytotoxicity analysis
The cytotoxicity of the tested extracts was evaluated with the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) test, using the MTT Cell Proliferation Assay Kit (Thermofisher, USA). The test principle is based on live cells ability to reduce orange tetrazolium salt to water-insoluble purple formazan crystals. Cells were inoculated into 96-well plate in the concentration of 2.5 × 105 cells/mL. Tested compounds were added when 70–80% confluence was achieved. MTT solution (4 mg/mL) was added to the culture after 24 and 48 h of incubation with extracts. After following 4-h incubation, the medium with MTT salt was removed and then the formed crystals were dissolved in dimethyl sulfoxide. The solution absorbance was measured at 540 nm, using PowerWave™ microplate spectrophotometer (Bio-Tek Instruments, USA). Each experiment was conducted three times and was measured in triplicates.
mRNA expression
Cells were inoculated into a 6-well plate in the concentration of 2.5 × 105 cells/mL. Tested compounds were added when 70–80% confluence was achieved. After 24 and 48 h, incubation cells were harvested using trypsin. RNA was isolated from the cells using Syngen Blood/Cell RNA Mini Kit (Syngen Biotech, Poland) according to the manufacturer’s protocol. cDNA was synthesised using High Capacity cDNA Reverse Transcription Kit (Thermofisher, USA) following the manufacturer’s instructions. Quantitative real-time PCR was performed in triplicate using TaqMan® Gene Expression Assays: Hs01023894_m1 (CDH1), Hs00153133_m1 (COX2) and Hs02758991_g1 (GAPDH) (Thermofisher, USA) with 7500 Fast Real-Time PCR system (Applied Biosystem, USA). Relative gene expression levels were determined using ΔΔCt method and presented as the mean fold change (RQ = 2− ΔΔCt). GAPDH was used as a reference gene.
Protein expression
Cells were inoculated into a 6-well plate in concentration of 2.5 × 105 cells/mL. Tested compounds were added when 70–80% confluence was achieved. After 24- and 48-h incubation, the cells were washed with PBS and then were incubated in lysis buffer (150 mM NaCl, 1% Triton X-100, 0.1%SDS, 50 mM Tris pH = 8) for 30 min in 4°C. The lysates were centrifuged (20 min, 12,000 rpm), and the protein concentration in the supernatants was determined by the Bradford method. The extracted protein (20 μg) was loaded in a polyacrylamide gel (NuPAGE Bis-Tris Gels, Invitrogen, USA) and 50-min electrophoretic separation was performed under reducing conditions with XCellSureLock instrument (Invitrogen, USA) at a constant voltage of 200 V. After separation, the gel and the nitrocellulose membrane (Invitrogen, USA) were placed between two layers of the filter paper and put between two electrodes of the XCell II Blot Module electrotransfer apparatus (Invitrogen, USA). The transfer was conducted for 60 min at a constant voltage of 30 V. Blots were developed using Western Breeze Chromogenic Detection Kit (Invitrogen, USA). Nonspecific antibody-binding sites were blocked and the membrane was incubated in the solution of the primary antibody: CDH1 antibody (H-108), COX2 antibody (H-62) and β-actin antibody (N-21) (Santa Cruz Biotechnology, US). After washing, the membrane was incubated with the secondary antibody combined with alkaline phosphatase. The last step was to mark the sites of reaction with antigen by the reaction of alkaline phosphatase and chromogen. The relative intensities of bands were quantified using the 1D Image Analysis Software program (Kodak, USA), and all the values were normalised to the intensities of the respective β-actin signal that was used as a loading control (Abcam, USA).
Statistical analysis
The results were analysed statistically in the STATISTICA vs. 13 application (StaftSoft, Poland). Data were calculated as mean ± SD. To compare two groups, Student’s t-test was used. To compare more than two groups, the one-way analysis of variance (ANOVA) and post hoc multiple comparisons on a basis of Tukey’s HSD test were used. All parameters were considered statistically significantly different if P values were less than 0.05.
Results
Toxicity of plant extracts
Cytotoxicity of the tested extracts was different for MKN28 and MKN74 cell lines. Allium angulosum and A. ursinum were toxic for MKN28 cells (48.60 ± 4.46 and 66.77 ± 3.00% viability after 48 h incubation respectively, see Table 1). All tested extracts revealed toxicity towards MKN74 cells; however, it was differentiated – the highest toxicity was observed after 48 h treatment with A. ursinum extract (31.55 ± 2.04% viability), and the lowest was observed after 48 h treatment with A. tibeticum (84.56 ± 6.60% viability) (Table 1).
Table 1. Treated MKN28 and MKN74 cells’ viability (% of control) after 24 and 48 h
| Garlic extract (0.3 μg/mL) |
MKN28 |
MKN74 |
| 24 h |
48 h |
24 h |
48 h |
| A. angulosum |
75.194 |
± |
7.482* |
48.598 |
± |
4.465*** |
40.147 |
± |
2.626 *** |
34.850 |
± |
2.797*** |
| A. sativum PL |
110.888 |
± |
16.681 |
93.474 |
± |
3.460 |
59.941 |
± |
4.602*** |
64.969 |
± |
2.604*** |
| A. sativum MY |
101.550 |
± |
4.234 |
100.431 |
± |
4.817 |
74.366 |
± |
0.721*** |
71.102 |
± |
1.877*** |
| A. ursinum |
61.135 |
± |
1.548*** |
66.775 |
± |
3.003*** |
35.339 |
± |
4.478*** |
31.546 |
± |
2.037*** |
| A. tibeticum |
87.174 |
± |
1.898 |
96.872 |
± |
10.001 |
74.336 |
± |
3.111*** |
84.582 |
± |
6.601** |
| A. lusitanicum |
86.786 |
± |
6.116 |
84.520 |
± |
2.049* |
86.903 |
± |
4.752** |
67.346 |
± |
7.153*** |
Abbreviations: A. – Allium; PL – Poland; MY – Malaysia; SD – standard deviation.
***P < 0.001
**P < 0.01
*P < 0.05 vs. relevant control (mean ± SD; MKN28 control 24 h: 100.000 ± 3.763; 48 h: 99.946 ± 2.73; MKN74 control 24 h: 100.029 ± 6.731; 48 h: 100.023 ± 7.941)
|
E-cadherin and cyclooxygenase 2 expression
In our study, four ethanol extracts of three Allium species (A. sativum PL and MY, A. ursinum and A. tibeticum) influenced both CDH1 and COX2 levels in MKN74 human gastric cell line (Figs. 1 and 2) in contrast to MKN28 cell line, where this correlation was not observed.
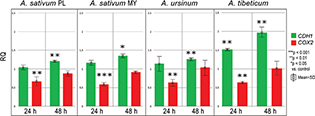
Fig 1. Chart bar illustrating the results of CDH1 and COX2 mRNA expression in MKN74 cell line treated with Allium sativum PL (Poland), Allium sativum MY (Malaysia), Allium ursinum and Allium tibeticum after 24 and 48 h. Data are presented as the mean RQ ± SD. The statistical significance of differences between control and treated cultured was presented as *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
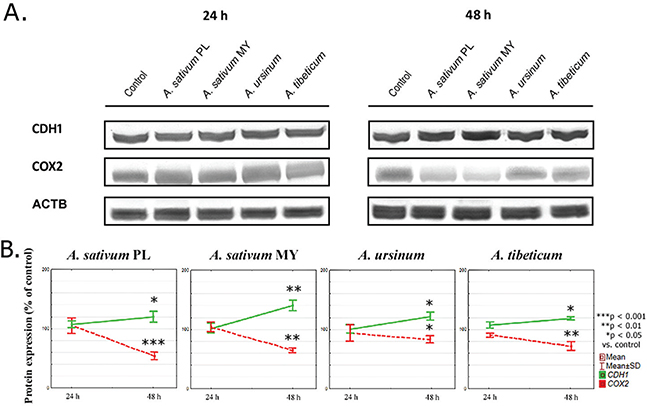
Fig 2. Graphs illustrating the results of: (A) Representative western blots from whole cell extract showing CDH1 and COX2 protein levels, and (B) CDH1 and COX2 protein expression. Data are presented as the mean percentage of control ±SD. The statistical significance of differences between control and treated cultured was presented as *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
mRNA expression
After 24 h of incubation, CDH1 mRNA expression was elevated after treating with A. sativum MY and A. tibeticum extracts (RQ = 1.17 ± 12 and 1.52 ± 0.03, respectively) in comparison to the control cells. After 48 h, the expression was even higher for A. sativum PL, A. sativum MY, A. tibeticum and A. ursinum extracts (see Fig. 1).
COX2 mRNA expression was considerably decreased at 24 h. At 48 h, it increased and did not exceed the control level for A. sativum PL ethanol extract (RQ = 0.88 ± 0.06) and A. sativum MY ethanol extract (RQ = 0.91 ± 0.05). In general, ethanol extracts influenced COX2 mRNA expression at 24 h, and the intensity of this effect decreased over time (Fig. 1).
Protein expression
To evaluate whether changes in mRNA expression were translated into protein expression, we conducted western blotting analysis. After 24 h of incubation, the proteins levels of both CDH1 and COX2 changed significantly after A. tibeticum extract treatment (107.48 ± 3.26 and 90.32 ± 3.67% respectively, Fig. 2). After 48 h, increased CDH1 expression and decreased COX2 expression were observed.
As one can compare, COX2 mRNA expression was decreased after 24 h in comparison to the control, then increased in time. On the contrary, the COX2 protein expression significantly lowered after 48 h when compared to the control level.
Influence of plant extracts on DOX toxicity
The week toxicity of DOX towards MKN74 cell line has been revealed (84.03 ± 2.88%). Selected extracts were even more toxic than DOX alone (A. sativum PL: 621.76 ± 2.34%; A. sativum MY: 77.10 ± 4.39%; A. ursinum: 39.57 ± 1.91%; A. tibeticum: 77.30 ± 0.83%) and their simultaneous treatment did not diminish observed toxicity. Moreover, synergistic effect of DOX and A. sativum (PL: 52.12 ± 2.83% and MY: 62.63 ± 2.31%) was observed (Fig. 3).
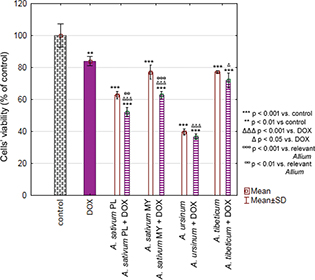
Fig 3. Chart bar illustrating the results of MTT assay carried out with MKN74 cell line treated with doxorubicin (DOX), Allium sativum PL (Poland), Allium sativum MY (Malaysia), Allium ursinum and Allium tibeticum as well as DOX + single extract after 24 h. Data are presented as the mean ± SD. Results were compared with control, DOX or relevant Allium extract.
Discussion
Epidemiologically, GC is one of the most common malignancies in humans. It is the sixth malignant tumour in incidence and the third in terms of mortality (1). Numerous studies have shown that vegetables from the Allium family, mainly garlic, may play an important role in the prevention of cancer (36, 37) and its treatment (38). One of the possible mechanisms of this phenomenon is the CDH expression restoration and thus inhibition of tumour invasion and metastasis (39) – CDH is an essential protein in the cell–cell adhesion and its downregulation and mutations favour cancer development and metastasis (40). This process has been previously reported in prostate and oral squamous cancer cells (41, 42). However, to the best of our knowledge, our study is the first concerning garlic-induced CDH restoration as a potential application in GC treatment.
The second molecule that plays an important role in the development and progression of GC is COX2. Its expression has been demonstrated to be elevated in this kind of tumour. For this reason, its inhibition has become one of the strategies of GC prevention and treatment. For the first time, our research team showed an inverse relationship between CDH and COX2 in GC (24). Garlic and other Allium species representatives are known anti-inflammatory agents and act as COX2 inhibitors. To our knowledge, the influence of garlic on COX2 expression as well as the relationship between CDH and COX2 after treatment with Allium extracts in GC has never been investigated. In our study, the CDH1 and COX2 expression depended on the time of exposure to garlic extracts. In all tested extracts, a positive effect of increased CDH1 expression intensified in 48 h. Largest drop in COX2 expression was observed in 24 h. These findings suggest that the effect of garlic extracts on GC cells is reversible. It is worth noting that the durability of this effect may depend on the tumour cell line – in Moden et al. (43) study, breast cancer cells supplemented with garlic extracts remained in growth arrest for 3 weeks.
The question is why the influence of the examined extracts was observed only in MKN74 cells. A possible explanation can be the difference in the COX2 expression level in both cell lines. As Saukkonen et al. (44) revealed, MKN74 cell line is characterised by higher COX2 expression than MKN28, both at the mRNA and protein levels, and thus is more sensitive. Therefore, it is possible that tumours with increased COX2 expression will be more susceptible to garlic treatment.
The pattern of gene expression is not always related to cytotoxicity. However, studies indicate that COX2 inhibitors are associated not only with chemoprophylactic effects but also with the chemotherapeutic potential in GC. Both COX-dependent and COX-independent pathways play a major role in the anti-tumour efficacy of COX2 inhibitors (31). For this reason, we evaluated the toxicity of all obtained extracts against the MKN28 and MKN74 cell lines and compared them with the observed inverse relationship between CDH and COX2. MKN28 cells were sensitive to A. angulosum and A. ursinum ethanolic extracts (Table 1). All tested extracts were toxic to MKN74 cells, of which A. angulosum and A. ursinum showed the strongest effect (Table 1). These results suggest that cytotoxicity was independent of changes in the CDH1 and COX2 expression patterns. There have been reports on the anti-tumour activity of A. sativum and A. ursinum (45–48); however, for the first time we revealed the cytotoxic activity of A. angulosum on GC.
DOX is an anthracycline chemotherapy agent that has been used as the gold standard for advanced GC since 1980 (4). However, DOX-based treatment is not recommended in chemotherapy for GC because of the frequent development of resistance and poor drug efficacy. The study assessed the effect of the selected extract on the toxicity of DOX relative to the MKN74 cell line. It is believed that DOX exerts anti-tumour activity through two different pathways – intercalation within DNA base pairs causing DNA strands breakage as well as the production of reactive oxygen species (ROS) that cause death in both cancer and normal cells. Oxidative stress is more often considered in the context of anthracycline cardiotoxicity (31, 49), and many studies have shown cardioprotective activity of plants from Allium species, especially garlic (50). However, their effect on DOX anti-cancer activity has not been studied. Allium species belong to natural antioxidants, and the effect of this group of compounds on the anticancer drug, including DOX, remains controversial (51). Because of the DOX oxidation mechanism, we could suppose weakening the antitumor activity of the drug. On the other hand, synergistic effects of DOX on antioxidants have been noticed. Our study showed that the ethanol extracts of A. tibeticum and A. ursinum were more toxic than DOX alone and the concomitant treatment did not affect its toxicity. In addition, A. sativum (PL and MY) showed a synergistic effect with the clinical dose of DOX.
This suggests that ROS production is not critical to DOX anti-tumour activity in this situation. This is consistent with previous observations that ROS-induced apoptosis of tumour cells occurs only in supraclinical doses of anthracyclines (52).
Conclusions
Our results indicate the potential application of Allium genus to chemoprevention and treatment support through CDH1 restoration and COX2 downregulation. However, the issue needs further in vivo investigations as in the future it might be used in clinics, possibly as a supplement to the chemotherapy. Moreover, the tested extracts revealed cytotoxic properties against GC cell lines and they had beneficial effect on DOX treatment.
Acknowledgements
The authors thank prof. Johan Offerhaus from the Department of Pathology, University Medical Center, Utrecht, the Netherlands, for the provision of MKN28 and MKN74 cell lines.
Data availability
The data sets used and/or analysed during this study are available from the corresponding author on reasonable request.
References
- WHO (2018). Cancer. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer [cited 25 January 2019].
- Nagini S. Carcinoma of the stomach: a review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol 2012; 4(7): 156–69. doi: 10.4251/wjgo.v4.i7.156.
- Shi J, Qu JP, Hou P. Pathogenetic mechanisms in gastric cancer. World J Gastroenterol 2014]; 20(38): 13804–19. doi: 10.3748/wjg.v20.i38.13804.
- MacDonald JS, Schein PS, Woolley PV, Smythe T, Ueno W, Hoth D, et al. 5-Fluorouracil, doxorubicin, and mitomycin (FAM) combination chemotherapy for advanced gastric cancer. Ann Intern Med 1980; 93: 533–6. doi: 10.7326/0003-4819-93-4-533.
- Chese MW, Reveal J, Fay MF. A subfamilial classification for the expanded asparagalean families amaryllidaceae, asparagaceae and xanthorrhoeaceae. Bot J Linean Soc 2009; 161: 132–6. doi: 10.1111/j.1095-8339.2009.00999.x.
- Salunkhe VN, Anandhan S, Gawande SJ, Ikkar RB, Bhagat YS, Mahajan V. First report of Colletotrichum truncatum causing anthracnose of mouse garlic (Allium angulosum) in India. Plant Dis 2018; 102(1): 240. doi: 10.1094/PDIS-04-17-0566-PDN
- Pittler MH, Ernst E. Clinical effectiveness of garlic (Allium sativum). Mol Nutr Food Res 2007; 51: 1382–5. doi: 10.1002/mnfr.200700073.
- Stajner D, Igić R, Popović BM, Malencić DJ. Comparative study of antioxidant properties of wild growing and cultivated Allium species. Phytother Res 2008; 22(1): 113–17. doi: https://doi.org/10.1002/ptr.3394.
- Herman-Antosiewicz A, Powolny AA, Singh SV. Molecular targets of cancer chemoprevention by garlic-derived organosulfides. Acta Pharmacol Sin 2007; 28(9): 1355–64 doi: 10.1111/j.1745-7254.2007.00682.x.
- Tsai CW, Chen HW, Sheen LY, Lii CK. Garlic: Health benefits and actions. BioMedicine. Elsevier Taiwan LLC 2012; 2(1): 17–29. doi: 10.1016/j.biomed.2011.12.002.
- Bommareddy A, VanWert AL, McCune DF, Brozena SL, Witczak Z, Singh SV. The role of organosulfur compounds derived from Allium vegetables in cancer prevention and therapy. In: Ullah M, Ahmad A, eds. Critical dietary factors in cancer chemoprevention. Cham: Springer; 2016, pp. 111–52. Available from: https://link.springer.com/chapter/10.1007/978-3-319-21461-0_6 [cited 25 January 2019]. doi: 10.1007/978-3-319-21461-0_6.
- Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res 1997; 57: 1276–80. Available form: http://cancerres.aacrjournals.org/content/57/7/1276.long [cited 25 January 2019].
- Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, et al. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res 2000; 6(2): 519–25. Available from: http://clincancerres.aacrjournals.org/content/6/2/519.long [cited 25 January 2019].
- Mao XY, Wang XG, Lv XJ, Xu L, Han CB. COX-2 expression in gastric cancer and its relationship with angiogenesis using tissue microarray. World J Gastroenterol 2007; 13(25): 3466–71. doi: 10.3748/wjg.v13.i25.3466.
- Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J 1998; 12: 1063–73. Available from: https://pdfs.semanticscholar.org/4e0d/1583138af91b67a68f7ea55a5d405ce25b1c.pdf [cited 25 January 2019].
- Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998; 93: 705–16. doi: 10.1016/S0092-8674(00)81433-6.
- Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, et al. Cyclooxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol 2001; 2: 544–51. doi: 10.1016/S1470-2045(01)00488-0.
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 2005; 6: 622–34. doi: 10.1038/nrm1699.
- Winter JM, Ting AH, Vilardell F, Gallmeier E, Baylin SB, Hruban RH, et al. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res 2008; 14: 412–18. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3810144/ doi: 10.1158/1078-0432.CCR-07-0487 [cited 25 January 2019].
- Dohadwala M, Yang SC, Luo J, Sharma S, Batra RK, Huang M, et al. Cyclooxygenase-2-dependent regulation of E-cadherIn: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res 2006; 66(10): 5338–45. doi: 10.1158/0008-5472.CAN-05-3635.
- Bozzo F, Bassignana A, Lazzarato L, Boschi D, Gasco A, Bocca C, et al. Novel nitro-oxy derivatives of celecoxib for the regulation of colon cancer cell growth. Chem Biol Interact 2009; 182: 183–90. doi: 10.1016/j.cbi.2009.08.006.
- Jang TJ, Cha WH, Lee KS. Reciprocal correlation between the expression of cyclooxygenase-2 and E-cadherin in human bladder transitional cell carcinomas. Virchows Arch 2010; 457: 319–28. doi: 10.1007/s00428-010-0943-3.
- Fujii R, Imanishi Y, Shibata K, Sakai N, Sakamoto K, Shigetomi S, et al. Restoration of E-cadherin expression by selective Cox-2 inhibition and the clinical relevance of the epithelial-to-mesenchymal transition in head and neck squamous cell carcinoma. J Exp Clin Cancer Res 2014; 33: 40. doi: 10.1186/1756-9966-33-40.
- Sitarz R, Leguit RJ, de Leng WW, Morsink FH, Polkowski WP, Maciejewski R, et al. Cyclooxygenase-2 mediated regulation of E-cadherin occurs in conventional but not early-onset gastric cancer cell lines. Cell Oncol 2009; 31: 475–85. doi: 10.3233/CLO-2009-0496.
- Saukkonen K, Rintahaka J, Sivula A, Buskens CJ, Van Rees BP, Rio MC, et al. Cyclooxygenase-2 and gastric carcinogenesis. 2003; APMIS 111: 915–25. doi: 10.1034/j.1600-0463.2003.1111001.x.
- Wang GY, Lu CQ, Zhang RM, Hu XH, Luo ZW. The E-cadherin gene polymorphism −160C→A and cancer risk: a HuGE review and meta-analysis of 26 case-control studies. Am J Epidemiol 2008; 167(1): 7–14. Available from: http://aje.oxfordjournals.org/content/167/1/7.abstract [cited 25 January 2019]. doi: 10.1093/aje/kwm264
- Goldberg Y, Nassif II, Pittas A, Tsai LL, Dynlacht BD, Rigas B, et al. The anti-proliferative effect of sulindac and sulindac sulfide on HT-29 colon cancer cells: alterations in tumor suppressor and cell cycle-regulatory proteins. Oncogene 1996; 12: 893–901.
- Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med 1999; 5: 1418–23. Available from: https://www.nature.com/articles/nm1299_1418 [cited 25 January 2019].
- Takahashi T, Kozaki K, Yatabe Y, Achiwa H, Hida T. Increased expression of COX-2 in the development of human lung cancers. J Environ Pathol Toxicol Oncol 2002; 21: 177–81.
- Muller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Furstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci U S A 2002; 99: 12483–8. doi: 10.1073/pnas.192323799.
- Wang Z, Chen J, Liu J. COX-2 inhibitors and gastric cancer. Gastroenterol Res Pract. 2014; 2014: 132320. doi: 10.1155/2014/132320.
- Kim J, Shim M. COX-2 inhibitor NS-398 suppresses doxorubicin-induced p53 accumulation through inhibition of ROS-mediated Jnk activation. Mol Carcinog 2016; 55(12): 2156–67. doi: 10.1002/mc.22458.
- Zeng Y, Li Y, Yang J, Pu X, Du J, Yang X, et al. Therapeutic role of functional components in alliums for preventive chronic disease in human being. Evid Based Complement Alternat Med 2017; 2017: 9402849. doi: 10.1155/2017/9402849.
- Ali M. Mechanism by which garlic (Allium sativum) inhibits cyclooxygenase activity. Effect of raw versus boiled garlic extract on the synthesis of prostanoids. Prostaglandins Leukot Essent Fatty Acids 1995; 53(6): 397–400.
- Segredo MP, Salvadori DM, Rocha NS, Moretto FC, Correa CR, Camargo EA, et al. Oxidative stress on cardiotoxicity after treatment with single and multiple doses of doxorubicin. Hum Exp Toxicol 2014; 33: 748–60. doi: 10.1177/0960327113512342.
- Turati F, Pelucchi C, Guercio V, La Vecchia C, Galeone C. Allium vegetable intake and gastric cancer: a case-control study and meta-analysis. Mol Nutr Food Res 2015; 59(1): 171–9. doi: 10.1002/mnfr.201400496.
- Kodali RT, Eslick GD. Meta-analysis: does garlic intake reduce risk of gastric cancer? Nutr Cancer 2015; 67(1): 1–11. doi: 10.1080/01635581.2015.967873.
- Lamm DL, Riggs DR. The potential application of Allium sativum (garlic) for the treatment of bladder cancer. Urol Clin North Am 2000; 27(1): 157–62,xi. Available from: https://www.sciencedirect.com/science/article/pii/S0094014305702433?via%3Dihub [cited 25 January 2019]. doi: 10.1016/S0094-0143(05)70243-3
- Tafrihi M, Nakhaei Sistani R. E-Cadherin/β-catenin complex: a target for anticancer and antimetastasis plants/plant-derived compounds. Nutr Cancer 2017]; 69(5): 702–22. doi: 10.1080/01635581.2017.1320415.
- Chen HC, Chu RY, Hsu PN, Hsu PI, Lu JY, Lai KH, et al. Loss of E-cadherin expression correlates with poor differentiation and invasion into adjacent organs in gastric adenocarcinomas. Cancer Lett. Ireland. 2003; 201(1): 97–106. doi: 10.1016/j.canlet.2003.07.007.
- Chu Q, Ling MT, Feng H, Cheung HW, Tsao SW, Wang X, et al. A novel anticancer effect of garlic derivatives: inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis. England 2006; 27(11): 2180–9. doi: 10.1093/carcin/bgl054
- Tang FY, Chiang EPI, Chung JG, Lee HZ, Hsu CY. S-allylcysteine modulates the expression of E-cadherin and inhibits the malignant progression of human oral cancer. J Nutr Biochem 2009]; 20(12): 1013–20. doi: 10.1016/j.jnutbio.2008.09.007.
- Moden S, Dicarlo SE, Reddy TR. Fresh garlic extract induces growth arrest and morphological differentiation of MCF7 breast cancer cells. Genes Cancer 2012; 3(2): 177–86. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3463925&tool=pmcentrez&rendertype=abstract [cited 25 January 2019]. doi: 10.1177/1947601912458581
- Saukkonen K, Nieminen O, van Rees B, Vilkki S, Härkönen M, Juhola M, et al. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin Cancer Res 2001; 7(7): 1923–31. Available from: http://clincancerres.aacrjournals.org/content/7/7/1923.long [cited 25 January 2019].
- Ghazanfari T, Yaraee R, Rahmati B, Hakimzadeh H, Shams J, Jalali-Nadoushan MR. In vitro cytotoxic effect of garlic extract on malignant and nonmalignant cell lines. Immunopharmacol Immunotoxicol 2011; 33(4): 603–8. Available from: http://www.tandfonline.com/doi/full/10.3109/08923973.2011.551832 [cited 25 January 2019]. doi: 10.3109/08923973.2011.551832
- Matsuura N, Miyamae Y, Yamane K, Nagao Y, Hamada Y, Kawaguchi N, et al. Aged garlic extract inhibits angiogenesis and proliferation of colorectal carcinoma cells. J Nutr 2006; 136(3 Suppl): 842S–6S. doi: 10.1093/jn/136.3.842S.
- Chandra-Kuntal K, Lee J, Singh SV. Critical role for reactive oxygen species in apoptosis induction and cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic. Breast Cancer Res Treat. 2013; 138(1): 69–79. doi: 10.1007/s10549-013-2440-2.
- Xu X, Song G, Yu Y, Ma H, Ma L, Jin Y. Apoptosis and G2/M arrest induced by Allium ursinum (ramson) watery extract in an AGS gastric cancer cell line. Onco Targets Ther 2013; 6: 779–83. doi: 10.2147/OTT.S45865
- Tsubura A, Lai YC, Kuwata M, Uehara N, Yoshizawa K. Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anticancer Agents Med Chem. 2011; 11(3): 249–53. doi: 10.2174/187152011795347441.
- Tende JA, Ayo JO, Mohammed A, Zezi AU. Blood pressure lowering and cardio-protective effects of garlic (Allium sativum) and ginger (Zingiber officinale) extracts in some laboratory animals. Int J Med Med Sci 2015; 7(1): 8–13. Available from: https://academicjournals.org/journal/IJMMS/article-full-text-pdf/F49ACB050204 [cited 25 January 2015]. doi: 10.5897/IJMMS2014.1069
- Mut-Salud N, Álvarez PJ, Garrido JM, Carrasco E, Aránega A, Rodríguez-Serrano F. Antioxidant intake and antitumor therapy: toward nutritional recommendations for optimal results. Oxid Med Cell Longev 2016; 2016: 6719534. doi: 10.1155/2016/6719534.
- Panchuk R, Skorokhyd N, Chumak V, Lehka L, Omelyanchik S, Gurinovich V, et al. Specific antioxidant compounds differentially modulate cytotoxic activity of doxorubicin and cisplatIn: in vitro and in vivo study. Croat Med J 2014; 55(3): 206–17. doi: 10.3325/cmj.2014.55.206.