ORIGINAL ARTICLE
Impact of cooking on the antioxidant activity of spice turmeric
Jian-Long Sun1,2, Hong-Fang Ji1,2 and Liang Shen1,2*
1Institute of Biomedical Research, Shandong University of Technology, Zibo, Shandong, People’s Republic of China;
2Zibo Key Laboratory for Neurodegenerative Diseases Drug Development, Shandong Provincial Research Center for Bioinformatic Engineering and Technique, School of Life Sciences, Shandong University of Technology, Zibo, Shandong, People’s Republic of China
Popular scientific summary
- Curcuminoids are the main ingredients of spice turmeric, which is usually heated during cooking.
- The present study indicated that the cooked curcuminoids still possessed antioxidant activity.
Abstract
Curcuminoids, as the main ingredient of turmeric, are popularly used in food additives and condiments, and are widely accepted to be beneficial for human health for their antioxidant activity. However, curcuminoids are highly susceptible in terms of thermal-induced degradation, and curry is usually boiled, roasted, or fried in the use of food additives and condiments. Thus, it is interesting to explore the effect of cooking on the antioxidant activity of curcuminoids. In the present study, the total antioxidant capacity (T-AOC) of cooked curcuminoids (boiled curcuminoids, roasted curcuminoids, and fried curcuminoids) processed through three heating conditions, and their protective effects against oxidative damage to rat pheochromocytoma (PC12) cells, a well-established neuronal model, were evaluated. It was found that cooking slightly lowered the T-AOC of curcuminoids, with boiled curcuminoids being relatively stronger than roasted curcuminoids, and fried curcuminoids being the weakest form. Both boiled and roasted curcuminoids could significantly improve cell viability, mitigate intracellular accumulation of reactive oxygen species and reduce malondialdehyde activity, reduce caspase-3 and caspase-9 protein expression, and increase superoxide dismutase activity of PC12 cells compared with the control group. In comparison with parent curcuminoids, the protective effects of cooked curcuminoids got relatively lower overall, with boiled curcuminoids being relatively stronger than roasted curcuminoids. In conclusion, the cooked curcuminoids, including boiled and roasted forms, still have antioxidant and neuroprotective activity.
Keywords: curcuminoids; boil; roast; antioxidant activity; oxidative stress; PC12 cells
Citation: Food & Nutrition Research 2019, 63: 3451 - http://dx.doi.org/10.29219/fnr.v63.3451
Copyright: © 2019 Jian-Long Sun et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 27 January 2019; Revised: 5 March 2019; Accepted: 11 March 2019; Published: 31 May 2019
Competing interests and funding: The authors declare no conflict of interest. The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
*Liang Shen, Shandong Provincial Research Center for Bioinformatic Engineering and Technique, School of Life Sciences, Shandong University of Technology, Zibo 255049, People’s Republic of China. Email: shen@sdut.edu.cn
As the main ingredient of spice turmeric, curcuminoids are popularly used as food additives. Curcuminoids are responsible for the bright-yellow color of turmeric and thus are also used as a food coloring. It is widely recognized that curcuminoid supplementation is beneficial to human health and prevents many types of diseases in view of their multiple biological activities, including antioxidant, antiinflammatory, antibacterial, antiviral, antidiabetic, anticancer effects, etc. (1–4).
As we know, when curcuminoids are used as food additives or spice, they are usually processed by boiling or frying in oil during cooking. However, curcuminoids have the characteristic of low stability and degrade readily (5–8). Thus, it is interesting to evaluate the biological activities of boiled and roasted curcuminoids by comparing with the parent form.
Curcuminoids are natural antioxidants and can attenuate oxidative stress through scavenging reactive oxygen species (ROS) (9–11). The antioxidant activity of curcumin is a primary mechanism that explains the majority of their beneficial effects. In recent years, a series of in vitro and in vivo animal models, as well as epidemiological studies, have supported the benefits of curcumin in preventing and combating Alzheimer’s disease (AD) (12–19). Oxidative stress-induced cell injury via apoptosis or necrosis is regarded as the principal pathogenic factor of AD and other neurodegenerative diseases (20–22). Therefore, in this study, three conditions were employed to mimic boiling and roasting of curcuminoids during cooking. We studied the total antioxidant capacity (T-AOC) of boiled curcuminoids, roasted curcuminoids and fried curcuminoids, and the protective effects on the oxidative damage to PC12 cells.
Materials and methods
Materials
The rat pheochromocytoma PC12 cell line was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The reagents for cell culture, including penicillin–streptomycin, Nutrient Mixture F-12 Ham were purchased from Sigma (St. Louis, MO, USA). Horse serum (HS) and fetal bovine serum (FBS) were from Gibco (import). H2O2 was purchased from Shuangshuang Chemical Co., Ltd. (Yantai, China). Curcuminoids (mainly containing curcumin, Curcuminoids contain a mixture of curcumin; demethoxycurcumin and bidemethoxycurcumin. If available mention their proportion; How does it differ from pure curcumin that contains 98% curcuminoids? demethoxycurcumin, and bidemethoxycurcumin) were purchased from Shanghai Macklin biochemical science and Technology Co., Ltd. 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT), poly-D-lysine hydrobromide, and 2′,7′-dichlorofluorescindiacetate (DCFH-DA) were purchased from Sigma-Aldrich Shanghai Trading Co., Ltd. (Shanghai, China). The T-AOC assay kit was purchased from Beyotime Biotechnology (Shanghai, China). The assay kits for malondialdehyde (MDA), superoxide dismutase (SOD), caspase-3, and caspase-9 were purchased from Nanjing Bibo Biological Technology (Nanjing, China).
Preparation of boiled curcuminoids, roasted curcuminoids, and fried curcuminoids
The preparation of boiled curcuminoids, roasted curcuminoids, and fried curcuminoids to mimic cooking was carried out as follows. PBS (0.01 M, pH 7.4) was used to dissolve curcuminoids to 1 mg/mL in sterilized centrifuge tubes, and then heated for 1 h in water at 100°C. Freeze drier was used to obtain drug powder, and then was dissolved in dimethyl sulfoxide (DMSO), and finally filtered by millipore filters for further experiment. As for roasted curcuminoids, curcuminoids were roasted at 200°C for 1 h with an electric heating pan dryer, and then the drug powder was dissolved in DMSO, and filtered by 0.22 μm millipore filters for further study. Edible blending oil was used to dissolve curcuminoids to 1 mg/mL in sterilized glass tubes and then heated at 150°C for 10 min with collector type constant temperature heating magnetic agitator, to prepare the fried curcuminoids sample.
Total antioxidant capacity assay
The T-AOC of curcuminoids, boiled curcuminoids, roasted curcuminoids, and fried curcuminoids, was estimated with ferric reducing ability of plasma (FRAP) method. After preparation of the FRAP reagent, it was incubated at 37°C and was used within 2 h. A total of 5 μl of standard solution at a range of concentrations or tested sample was added to the FRAP reagent for 5 min at 37°C. Finally, the absorbance value was measured by the Multiwell microplate reader (MultiskanGo, Thermo Fisher Scientific, Vantaa, Finland) to calculate the T-AOC of the samples.
Cell viability assay
PC12 cells were cultivated in Nutrient Mixture F-12 Ham media supplemented with 10% HS, 5% FBS, 1% (v/v) penicillin and streptomycin at a temperature of 37°C with 5% CO2. Cytoprotective activity of curcuminoids, boiled curcuminoids, and roasted curcuminoids on cell injury caused by H2O2 was assessed by MTT assay (18).
ROS assay
The intracellular ROS level was estimated by means of a DCFH-DA assay. PC12 cells were pretreated with curcuminoids, boiled curcuminoids, and roasted curcuminoids for 0.5 h and then H2O2 was added for additional 6 h. Then, the PC12 cells were incubated with serum-free medium containing 10 μM DCFH-DA for 0.5 h. The relative fluorescence intensity of cells was estimated with a fluorescent microplate reader (Varioskan Flash, Thermo Fisher Scientific Oy, Vantaa, Finland), and then pictures were taken under a fluorescence microscope (Olympus IX73, Tokyo, Japan).
MDA, SOD, caspase-3, and caspase-9 assays
Cells were incubated in a 6-well plate for 24 h. Treatment with different concentrations of curcuminoids, boiled curcuminoids, and roasted curcuminoids on PC12 cells was same as the above assay. After 12 h, cells were washed with PBS and homogenized. The homogenate was centrifuged to collect the supernatant for further study. The MDA level and SOD, caspase-3 and caspase-9 activities, were determined by means of assay kits according to the manufacturer’s instruction.
Statistical analysis
All values obtained from three independent experiments were analyzed with SPSS (16.0) software and shown as the mean ± standard derivation (SD). The difference in different groups was compared and analyzed by one-way analysis of variance (ANOVA). The minimum significance level was defined as ##
P < 0.01, #
P < 0.05 versus control, and **P < 0.01, *P < 0.05 versus H2O2-treated cells.
Results
Total antioxidant capacity
The T-AOC of curcuminoids, boiled curcuminoids, roasted curcuminoids, and fried curcuminoids was measured using the FRAP method, and was characterized by mmol Fe2+ per milligram of dry weight. It was found that parent curcuminoids exhibited relatively higher T-AOC than the cooked forms. The T-AOC of boiled curcuminoids was found to be higher than roasted curcuminoids, with fried curcuminoids being the weakest form.
Protective effects of boiled and roasted curcuminoids
After testing different concentrations of H2O2 to damage the PC12 cells, we found that the survival rate of PC12 cells was 53.19 ± 2.96% at 500 μM concentration of H2O2. Therefore, the 500 μM concentration of H2O2 was used in further experiments as an oxidative injury model (Fig. 1a). According to the protection experiment, we found that curcuminoids (20 μg/mL), boiled curcuminoids (250 μg/mL), and roasted curcuminoids (40 μg/mL) have a stronger protective effect than the H2O2-only group, and increased the cell viability by 23.07 ± 4.42%, 18.91 ± 1.62%, and 13.72 ± 0.87%, respectively (Fig. 1b). Therefore, the three concentrations were used for all further experiments. Morphological cell image analysis indicated that PC12 cells exposed to 500 μM H2O2 for 24 h resulted in morphological alteration and cell number reduction, while pretreating with curcuminoids or the boiled and roasted curcuminoids attenuated the damages (Fig. 1c).
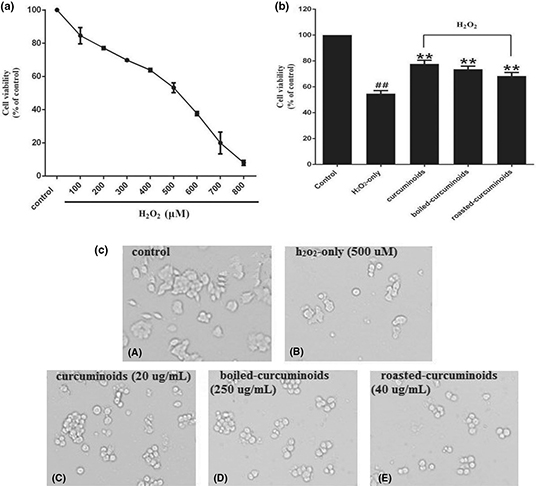
Fig. 1. Protective effects of curcuminoids, boiled curcuminoids, and roasted curcuminoids on PC12 cells against H2O2-induced damage. Cell viabilities treated with different concentrations of H2O2 (a). The protective effects of curcuminoids, boiled curcuminoids, and roasted curcuminoids on H2O2-induced cytotoxicity to PC12 cells (b). Morphological alteration of PC12 cells (c): (A) control cells; (B) cells treated with H2O2 only; cells pretreated with curcuminoids (20 μg/mL) (C), boiled curcuminoids (250 μg/mL) (D), roasted curcuminoids (40 μg/mL) (E), and co-treated with H2O2. Data were shown as mean ± SD (n = 3). (##P < 0.01 and #P < 0.05 vs. control; *P < 0.05 and **P < 0.01 vs. H2O2-treated cells).
Intracellular ROS
The intracellular ROS levels were estimated with the fluorescence probe DCFH-DA. In comparison with the control group, the intracellular ROS level in the oxidative injury group increased significantly to 194 ± 10.44% (Fig. 2a). PC12 cells pretreated with curcuminoids, boiled and roasted forms, significantly reduced ROS levels. Curcuminoids exhibited the most significant effect (143.67 ± 26.95%), followed by boiled and roasted curcuminoids (Fig. 2b).
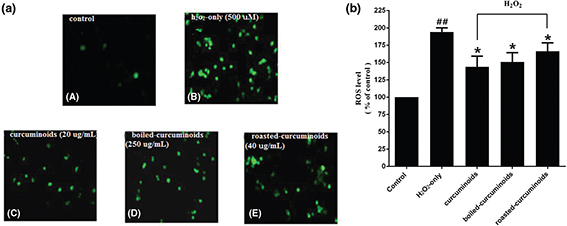
Fig. 2. Effects of curcuminoids, boiled curcuminoids, and roasted curcuminoids on intracellular ROS level. (a) Representative images of DCFH-DA staining in PC12 cells by the inverted fluorescence microscope. The labeling letters in this experiment were identical with Fig. 1C; (b) Intracellular ROS level. Data were shown as mean ± SD (n = 3). (##P < 0.01 and #P < 0.05 vs. control; *P < 0.05 and **P < 0.01 vs. H2O2-treated cells).
Table 1. Total antioxidant capacity of curcuminoids, boiled curcuminoids, roasted curcuminoids, and fried curcuminoids
| Compounds (1 mg/mL) |
Total antioxidant capacity (T-AOC) |
| Curcuminoids |
3.71 ± 0.18 |
| Boiled curcuminoids |
2.33 ± 0.04** |
| Roasted curcuminoids |
1.06 ± 0.16** |
| Fried curcuminoids |
0.212 ± 0.06** |
| Data were shown as mean ± SD (n = 3). (*P < 0.05 and **P < 0.01 vs. curcuminoids). |
MDA and SOD
PC12 cells exposed to H2O2 caused an increase in the intracellular MDA level and a decrease in the SOD activity. Pretreating cells with curcuminoids, boiled curcuminoids, or roasted curcuminoids for 0.5 h before being exposed to H2O2, clearly attenuated intracellular MDA level increase (Fig. 3a) and enhanced the SOD activity (Fig. 3b). The effect of boiled curcuminoids was relatively stronger than that of roasted curcuminoids.
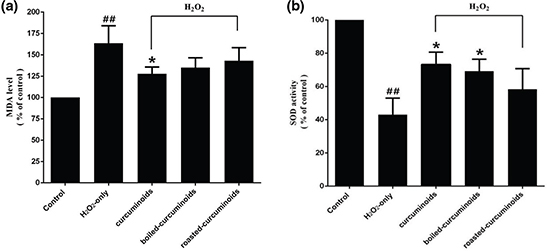
Fig. 3. Effects of curcuminoids, boiled curcuminoids, and roasted curcuminoids on the MDA level (a) and SOD activity (b) in PC12 cells caused by H2O2. Data were shown as mean ± SD (n = 3). (##P < 0.01 and #P < 0.05 vs. control; *P < 0.05 and **P < 0.01 vs. H2O2-treated cells).
Caspase-3 and caspase-9
Exposing PC12 cells to H2O2 resulted in an enhancement of caspase-3 and caspase-9 activities. Pretreatment with curcuminoids, boiled curcuminoids, and roasted curcuminoids effectively suppressed an increase in caspase-3 (Fig. 4a) and caspase-9 (Fig. 4b) activities caused by H2O2.
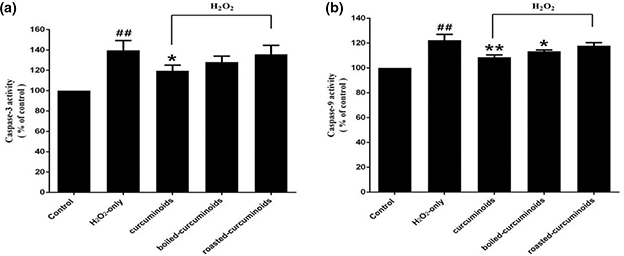
Fig. 4. Effect of curcuminoids, boiled curcuminoids, and roasted curcuminoids on caspase-3 (a) and caspase-9 (b) activities induced by H2O2. Data were shown as mean ± SD (n = 3). (##P < 0.01 and #P < 0.05 vs. control; *P < 0.05 and **P < 0.01 vs. H2O2-treated cells).
Discussion
Food processing can regulate biological activity of plant bioactive substances (23, 24). Turmeric, with curcuminoids as the main bioactive components, is a popular food additive and condiment. However, curcuminoids readily degrade when heated, and cooking like boiling and roasting will result in the degradation of curcuminoids to a great extent (5–7, 16). The degradation products of curcumin after cooking also possess biological properties similar to the parent compound (25, 26). Curcuminoids were reported to have neuroprotective effects via reducing oxidative stress (27). Herein, we studied the T-AOC, the protective effects of boiled and roasted curcuminoids on oxidative damage to PC12 cells. Boiled curcuminoids, roasted curcuminoids, and fried curcuminoids still possess T-AOC, and the capacity of fried curcuminoids was the weakest among the three forms. Both boiled and roasted curcuminoids could reduce the content of ROS, decrease the MDA level, and increase SOD activity. Previous studies indicated that the degradation products produced after heating, which retain the main functional groups of curcumin, contribute to the various pharmacological effects of curcuminoids (5–7, 16). The boiled and roasted curcuminoids also exhibited neuroprotective ability. This may provide potential clues to understand the reduced incidence of AD after consuming curcuminoids as food additives in daily life (17). In addition, it was also found that boiled curcuminoids possessed relatively stronger antioxidant activity than the roasted and the fried form, which may arise from two factors: 1) heating increased the solubility of boiled curcuminoids in PBS and 2) higher temperatures and oxygen exposure may destroy the antioxidant functional structures of roasted and fried curcuminoids to a certain extent.
Conclusions
In summary, the present study indicated that after processing with heating conditions mimicking cooking, boiled and roasted curcuminoids still possessed antioxidant capacities and could inhibit PC12 cellular injury induced by H2O2 through ameliorating oxidative stress, and the effect of boiled curcuminoids was relatively higher than the roasted form. More studies are encouraged to elucidate the molecular basis underlying the differences in neuroprotective activities between boiled and roasted curcuminoids.
Authors’ contributions
L.S. conceived and designed the experiments; J.L.S. performed the experiments; J.L.S., L.S., and H.F.J. analyzed the data; J.L.S., L.S., and H.F.J. wrote the paper.
Acknowledgements
This work was supported by the Shandong Provincial Natural Science Foundation (Grant Nos. ZR2018MH010 and JQ201508), and Shandong Provincial Key Research and Development Program (Grant No. 2018GSF121001), and Talent Program of Zibo.
References
- Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol 2008; 76: 1037–1636. doi: 10.1016/j.bcp.2008.08.008.
- Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci 2009; 30: 85–94. doi: 10.1016/j.tips.2008.11.002.
- Kunnumakkara AB, Sailo BL, Banik K, Harsha C, Prasad S, Gupta SC, et al. Chronic diseases, inflammation, and spices: how are they linked?. J Transl Med 2018; 16: 14. doi: 10.1186/s12967-018-1381-2.
- Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets 2011; 12: 332–347. doi: 10.2174/138945011794815356.
- Shen L, Ji HF. The pharmacology of curcumIn: is it the degradation products?. Trends Mol Med 2012; 18: 138–144. doi: 10.1016/j.molmed.2012.01.004.
- Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal 1997; 15: 1867–1876. doi: 10.1016/S0731-7085(96)02024-9.
- Lin JK, Pan MH, Lin-shiau SY. Recent studies on the biofunctions and biotransformations of curcumin. Biofactors 2000; 13: 153–158. doi: 10.1002/biof.5520130125.
- Ji HF, Shen L. Can improving bioavailability improve the bioactivity of curcumin?. Trends Pharmacol Sci 2014; 35: 265–266. doi: 10.1016/j.tips.2014.04.001.
- Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol 2007; 595: 105–125. doi: 10.1007/978-0-387-46401-53.
- Shen L, Ji HF. Theoretical study on physicochemical properties of curcumin. Spectrochim. Spectrochim Acta A Mol Biomol Spectrosc 2007; 67: 619–623. doi: 10.1016/j.saa.2006.08.018.
- Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic Biol Med 2007; 43: 568–580. doi: 10.1016/j.freeradbiomed.2007.05.009.
- Costa IM, Freire MAM, Cavalcanti JRLP, Araujo DP, Norrara B, RosaI MMM, et al. Supplementation with Curcuma longa reverses neurotoxic and behavioral damage in models of Alzheimer’s disease: a systematic review. Curr Neuropharmacol 2018; 16: 0. doi: 10.2174/0929867325666180117112610.
- Goozee KG, Shah TM, Sohrabi HR, Rainey-Smith SR, Brown B, Verdile G, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr 2016; 115: 449–465. doi: 10.1017/S0007114515004687.
- Tang M, Taghibiglou C. The mechanisms of action of curcumin in Alzheimer’s disease. J Alzheimers Dis 2017; 58: 1003–1016. doi: 10.3233/JAD-170188.
- Lim GP, Chu T, Yang F. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. Neuroscience 2001; 21: 8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001.
- Shen L, Liu CC, An CY, Ji HF. How does curcumin work with poor bioavailability? Clues from experimental and theoretical studies. Sci Rep 2016; 6: 20872. doi: 10.1038/srep20872.
- Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, et al. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology 2001; 57: 985–993. doi: 10.1212/WNL.57.6.985.
- Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, et al. Prevalence of dementia in an urban Indian population. Int Psychogeriatr 2001; 13: 439–450. doi: 10.1017/S1041610201007852.
- Marchiani A, Rozzo C, Fadda A, Delogu G, Ruzza P. Curcumin and curcumin-like molecules: from spice to drugs. Curr Med Chem 2014; 21: 204–222. doi: 10.2174/092986732102131206115810.
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006; 443: 787–795. doi: 10.1038/nature05292.
- Tonnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimer’s Dis 2017; 57: 1105–1121. doi: 10.3233/JAD-161088.
- Barnham KJ, Masters CL, Bush AI, Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 2004; 3: 205–214. doi: 10.1038/nrd1330.
- Boettler U, Sommerfeld K, Volz N, Pahlke G, Teller N, Somoza V, et al. Coffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expression. J Nutr Biochem 2011; 22: 426–440. doi: 10.1016/j.jnutbio.2010.03.011.
- Boettler U, Volz N, Pahlke G, Teller N, Kotyczka C, Somoza V, et al. Coffees rich in chlorogenic acid or N-methylpyridinium induce chemopreventive phase II-enzymes via the Nrf2/ARE pathway in vitro and in vivo. Mol Nutr Food Res 2011; 55: 798–802. doi: 10.1002/mnfr.201100115.
- Dahmke IN, Boettcher SP, Groh M, Mahlknecht U. Cooking enhances curcumin anti-cancerogenic activity through pyrolytic formation of ‘deketene curcumin’. Food Chem 2014; 26: 514–519. doi: 10.1016/j.foodchem.2013.11.102.
- Suresh D, Gurudutt KN, Srinivasan K. Degradation of bioactive spice compound: curcumin during domestic cooking. Eur Food Res Technol 2009; 228: 807–812. doi: 10.1007/s00217-008-0993-9.
- Hamaguchi T, Ono K, Yamada M. Review: curcumin and Alzheimer’s disease. CNS Neurosci Ther 2010; 16: 285–297. doi: 10.1111/j.1755-5949.2010.00147.x.